A composite preparation of novel coronavirus rbd protein fluorescent microspheres
A technology of composite preparations and fluorescent microspheres, which is applied in the field of saturated competition, can solve the problems of inability to perform detection and affect the performance of detection reagents, and achieve the effect of maintaining antigen activity and improving detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
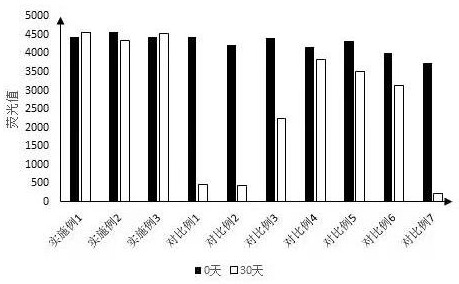
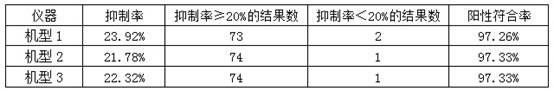
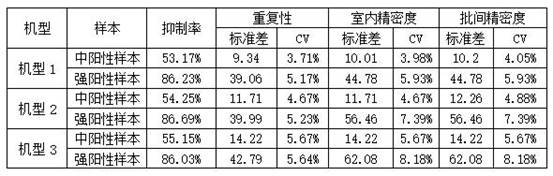
Image
Examples
Embodiment 1
[0052] A new crown RBD protein fluorescent microsphere composite preparation, the preparation method is as follows:
[0053] (1) Weigh 10mg ovalbumin and add it to phosphate buffered saline (0.02-0.1M PBS, pH 7.2-7.4), then add 10-50mg / mL 1-ethyl-(3-dimethylaminopropyl ) Carbodiimide (EDC), avoid light reaction for 15-30min;
[0054] (2) Weigh 10 mg of bovine serum albumin and 10 mg of hemocyanin into the activated ovalbumin solution;
[0055] (3) Dialyze with phosphate buffered saline (0.02-0.1M PBS, pH 7.2-7.4) to remove EDC;
[0056] (4) After affinity purification, the product whose molecular weight was the sum of the molecular weights of the three proteins was collected to obtain coupling protein 1 (BSA-OVA-KLH);
[0057] (5) Weigh 2.5g trehalose, 0.9g NaCl, 0.05g PEG4000, 0.05g Tween 20, 0.05gproclin 300, 0.24g Tris, 100g process water, add coupling protein 1 to make the final concentration 1%, adjust the pH to 8.5, to obtain the compound preparation.
Embodiment 2
[0059] A new crown RBD protein fluorescent microsphere composite preparation, the preparation method is as follows:
[0060] (1) Weigh 10mg bovine serum albumin and add it to phosphate buffered saline (0.02-0.1M PBS, pH 7.2-7.4), then add 10-50mg / mL 1-ethyl-(3-dimethylaminopropyl base) carbodiimide (EDC), avoid light reaction for 15-30min;
[0061] (2) Weigh 10 mg of silk fibroin and add it to the activated bovine serum albumin solution;
[0062] (3) Dialyze with phosphate buffered saline (0.02-0.1M PBS, pH 7.2-7.4) to remove EDC;
[0063] (4) After affinity purification, the product whose molecular weight was the sum of the molecular weights of the two proteins was collected to obtain coupling protein 2 (BSA-SF).
[0064] (5) Weigh 2.5g trehalose, 0.9g NaCl, 0.05g PEG4000, 0.05g Tween 20, 0.05gproclin 300, 0.24g Tris, 100g process water, add coupling protein 2 to make the final concentration 1%, adjust the pH to 8.5, to obtain the compound preparation.
Embodiment 3
[0066] A new crown RBD protein fluorescent microsphere composite preparation, the preparation method is as follows:
[0067] (1) Weigh 10mg of polylysine and add it to phosphate buffer (0.02-0.1M PBS, pH 7.2-7.4), then add 10-50mg / mL 1-ethyl-(3-dimethylaminopropyl base) carbodiimide (EDC), avoid light reaction for 15-30min;
[0068] (2) Weigh 10 mg bovine serum albumin and add it to the activated polylysine solution;
[0069] (3) Dialyze with phosphate buffered saline (0.02-0.1M PBS, pH 7.2-7.4) to remove EDC;
[0070] (4) After affinity purification, the product with higher protein concentration was collected to obtain coupling protein 3 (ε-PL-BSA);
[0071] (5) Weigh 5g sucrose, 1.3g MgCl 2 , 0.05g PEG4000, 0.02g Tetronin1307, 0.02g KroVin300M, 0.24g Tris, 100g process water, add coupling protein 3 to make the final concentration 1%, and adjust the pH to 8.5 to obtain a compound preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com