Recombinant collagen biphasic gel as well as preparation method and application thereof
A technology for recombining collagen and gel, which is applied in the fields of pharmaceutical formulation, tissue regeneration, medical science, etc., can solve the problems of wide particle size distribution, harsh process conditions, and difficult to clean thoroughly, so as to ensure the retention time and particle size. Highly controllable diameter and non-immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
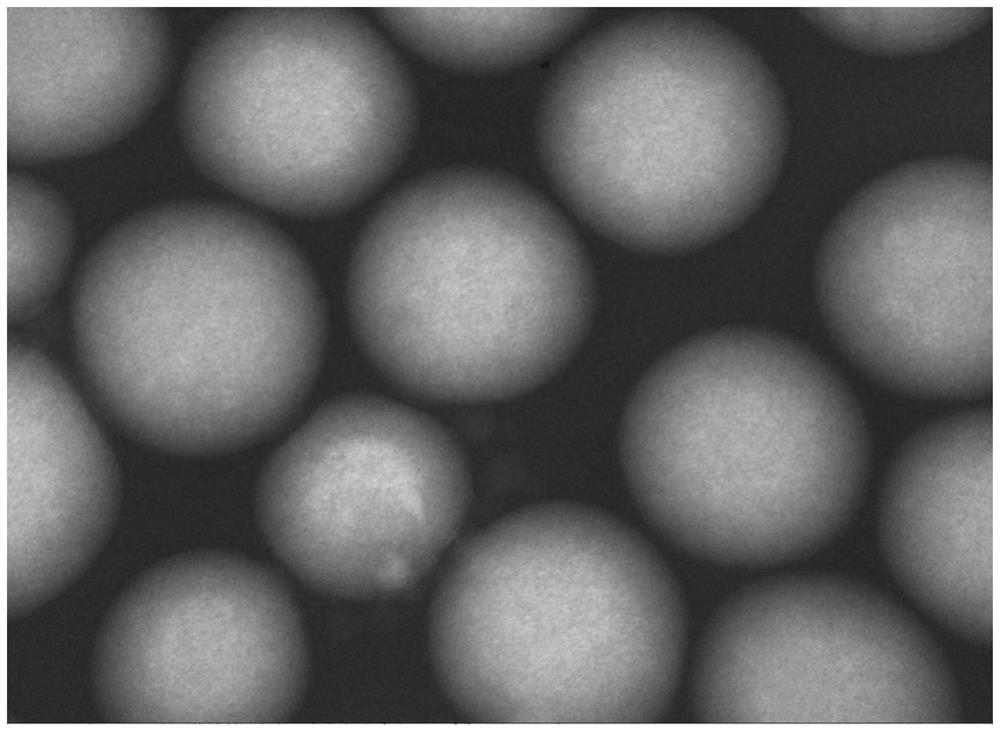
[0052] This embodiment provides a recombinant collagen biphasic gel, which includes recombinant collagen A microspheres and recombinant collagen B gel, wherein the recombinant collagen A microspheres are uniformly dispersed in the recombinant collagen B gel.
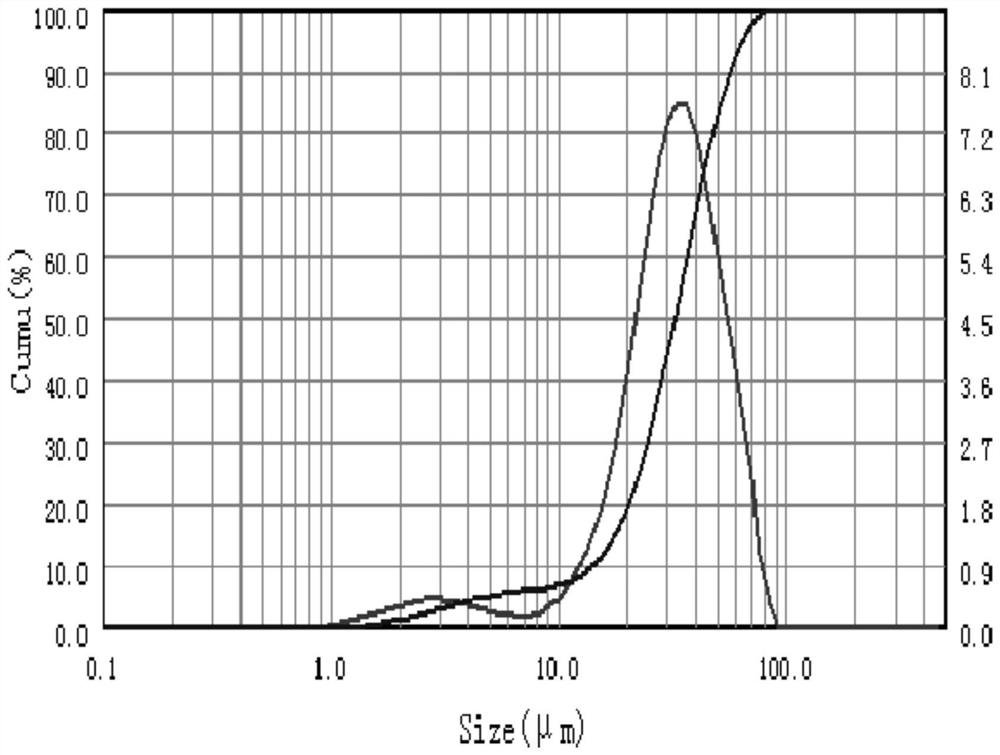
[0053] Wherein, the particle diameter of the recombinant collagen A microsphere is 20-35 μm, the molecular weight of the recombinant collagen A is 30KDa, and the molecular weight of the recombinant collagen B is 60KDa.
[0054] The preparation method of the above-mentioned recombinant collagen biphasic gel comprises the following steps:
[0055] S1: Dissolve recombinant collagen A (recombinant type I humanized collagen) in a phosphate buffer solution with a pH of 7 at room temperature to obtain a recombinant collagen A solution, and the concentration of the recombinant collagen A solution is 28mg / ml ;
[0056] S2: Take the recombinant collagen A solution as the water phase, add it to the liquid paraffin containing 4% Sp...
Embodiment 2
[0060] This embodiment provides a recombinant collagen biphasic gel, which includes recombinant collagen A microspheres and recombinant collagen B gel, wherein the recombinant collagen A microspheres are evenly dispersed in the recombinant collagen B gel; the recombinant collagen The mass percent content of the recombinant collagen A microspheres in the biphasic gel is 40%.
[0061] Wherein, the particle diameter of the recombinant collagen A microsphere is 40-60 μm, the molecular weight of the recombinant collagen A is 60KDa, and the molecular weight of the recombinant collagen B is 90KDa.
[0062] The preparation method of the above-mentioned recombinant collagen biphasic gel comprises the following steps:
[0063] S1: at room temperature, the recombinant collagen A (recombinant type I human-like collagen) was dissolved in a phosphate buffer solution with a pH of 7.0 to obtain a recombinant collagen A solution, and the concentration of the recombinant collagen A solution was...
Embodiment 3
[0069] This embodiment provides a recombinant collagen biphasic gel, which includes recombinant collagen A microspheres and recombinant collagen B gel, wherein the recombinant collagen A microspheres are evenly dispersed in the recombinant collagen B gel; the recombinant collagen The mass percent content of the recombinant collagen A microspheres in the biphasic gel is 25%.
[0070] Wherein, the particle diameter of the recombinant collagen A microsphere is 60-75 μm, the molecular weight of the recombinant collagen A is 100 KDa, and the molecular weight of the recombinant collagen B is 150 KDa.
[0071] The preparation method of the above-mentioned recombinant collagen biphasic gel comprises the following steps:
[0072] S1: At room temperature, recombinant collagen A (recombinant type III humanized collagen) was dissolved in a phosphate buffer solution with a pH of 5.5 to obtain a recombinant collagen A solution; wherein, the concentration of the recombinant collagen A soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com