An enzyme-linked immunoassay kit for human phosphorylated vasodilator-stimulating protein and its detection method
An enzyme-linked immunosorbent assay and vasodilation technology, applied in the field of molecular immunology, can solve the problems of complex process, high preparation cost, unfavorable large-scale industrial production, etc., and achieve the effects of simple detection steps, high sensitivity, and stable and repeatable results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the preparation of kit
[0039] The kit of the invention adopts the ELISA method and cooperates with a microplate reader, and is used for measuring the content of human phosphorylated blood vessel dilation-stimulated phosphoprotein in human body samples. The technical principle of the reaction is as follows: the VASP-P level in the serum is determined by the double-antibody sandwich method for the sample to be tested. Coat the microwell plate with VASP monoclonal antibody to make solid-phase antibody, divide the sample into two equal parts and treat them with activator and sample inhibitor respectively, add the VASP standard solution after sample treatment to the microwell coated with monoclonal antibody or sample solution, then add HRP-labeled phosphorylated VASP antibody to form antibody-antigen-enzyme-labeled antibody complex, after washing, add substrate TMB for color development; TMB is converted into blue under the catalysis of HRP enzyme, and in Un...
Embodiment 2
[0068] Embodiment 2, the test method of kit
[0069] (1) Take out the kit from the refrigerator and equilibrate to room temperature (18-25°C);
[0070] (2) Take sample activator dry powder and sample inhibitor dry powder and add 1ml sample pretreatment agent to dissolve;
[0071] (3) Take 10 μl sample activator and sample inhibitor respectively into the reaction well;
[0072] (4) Take 10 μl of the sample to be tested (whole blood) or the calibrator, add the sample activator and the sample inhibitor to the corresponding microwells of the microplate, mix well, and incubate at room temperature for 10 minutes;
[0073] (5) add to the corresponding microwell of the microplate, incubate, and carry out the first step reaction;
[0074] (6) Add HRP enzyme-labeled VASP monoclonal antibody, incubate in the dark, and carry out the second step of reaction;
[0075] (7) Wash, pat dry, add chromogenic solution, and incubate in the dark;
[0076] (8) Add stop solution to measure its abs...
Embodiment 3
[0078] Embodiment 3, the performance test of kit
[0079] (1) Use the kit of this application to pass the clinical sample verification
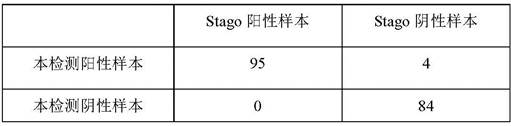
[0080] The kit of this application and the kits already on the market (CY-QUANTVASP / P2Y12, BIOCYTEX) detected 183 samples at the same time, and the data are shown in the following table:
[0081] The coincidence rate with the samples detected by Stago's VASP kit
[0082]
[0083] Negative coincidence rate: 84 / 88=95.45%;
[0084] Positive coincidence rate: 95 / 95=100%;
[0085] Total coincidence rate: 179 / 183=97.81%.
[0086] It can be seen from the above results that the human phosphorylated vasodilator-stimulated phosphoprotein ELISA kit of the present invention has good applicability and advancement.
[0087] (2) Linearity test of the kit
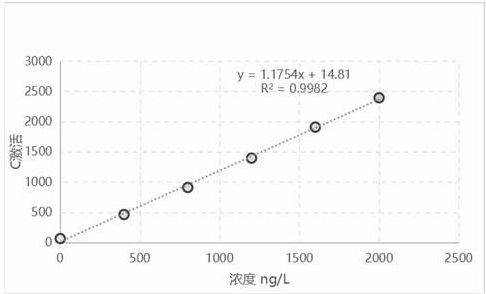
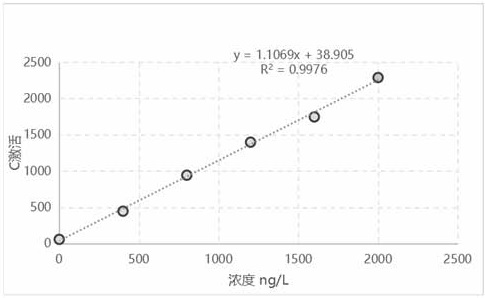
[0088] Draw the standard curve after using the protein calibrator to detect, the standard curve is as follows figure 1 , 2 As shown, the standard curve formula y (activation) = 1.1754x + 14.81, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com