New application of coumarin
A technology of coumarin and flavor regulator, applied in the new application field of coumarin, to achieve the effects of increasing insulin content, reducing expression, and high cell proliferation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
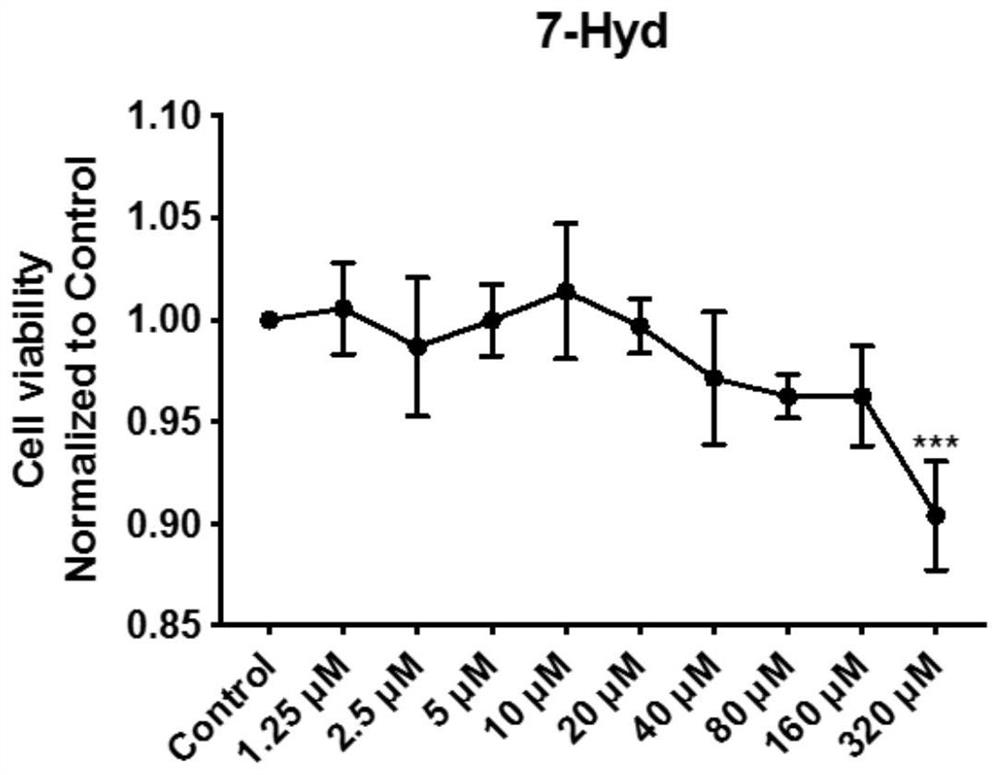
[0070] The MTT experiment involved in this embodiment, the main steps are: take GEnCs cells (glomerular endothelial cells) derived from the kidney in the logarithmic phase, and 5% CO 2 , 37°C for culture, followed by 5.0×10 per well 4 The cells were seeded into a 96-well plate, and a series of concentration gradients of 1.25 μM, 2.5 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, 160 μM, and 320 μM of coumarin (final concentration after adding to the well) were added, and the control group (Control ) without adding coumarin, at least 4 replicate wells for each concentration, after incubation for 24 hours, add 20 μL MTT solution to each well, continue to cultivate for 4 hours, carefully suck off the culture medium in the wells, add 150 μL dimethyl sulfoxide to each well, place Shake on a shaker for 10 minutes to fully dissolve the crystals. The absorbance of each well was measured at OD490nm in an enzyme-linked immunosorbent assay instrument.
[0071] The results show that coumarin has...
Embodiment 2
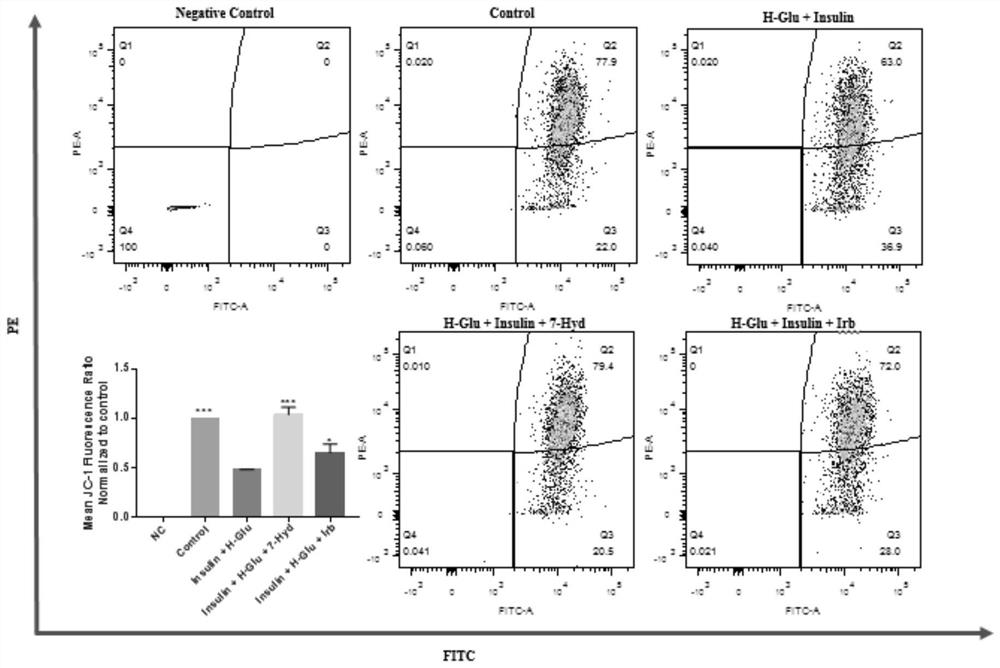
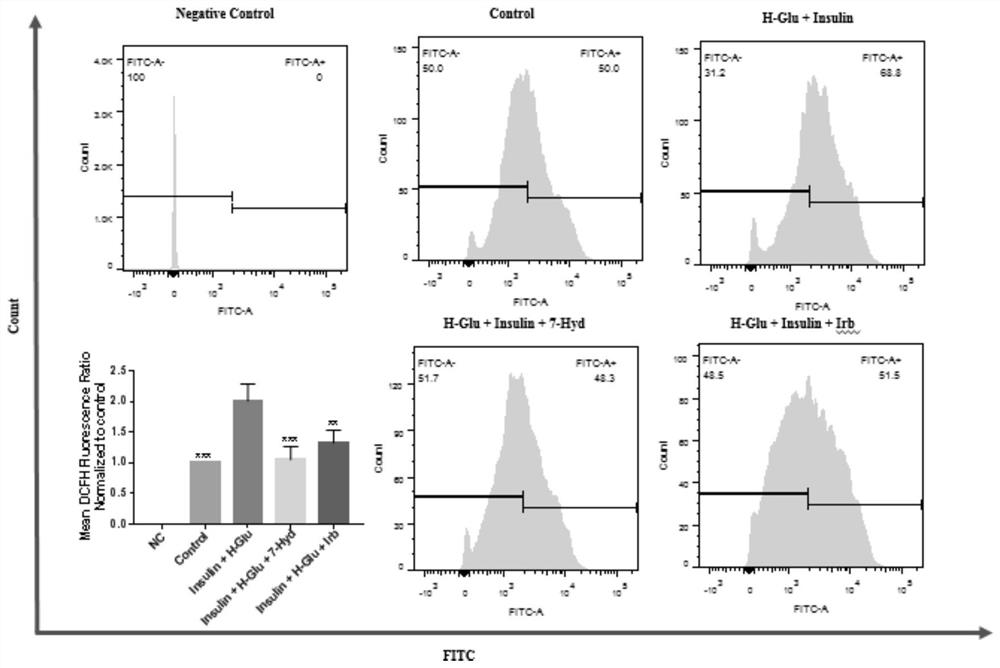
[0073] The flow cytometry involved in this embodiment, the main steps include:
[0074] Kidney-derived GEnCs cells were prepared at a density of 3.5×10 5Cells / mL were inoculated in a 6-well plate, and when the cells grew to 75% confluence, they were divided into negative control group (no treatment, pure cells without any treatment, deducting the self-fluorescence of cells, Negative Control), and blank control group (without adding any drugs, Control), model group (H-Glu+Insulin), coumarin group (H-Glu+Insulin+7-Hyd) and comparison drug group (H-Glu+insulin+Irb); wherein, Irb corresponds to irbesartan, the final concentration is 13.5mg / mL, and the final concentration of coumarin is 0.5mg / mL. Take it out of the incubator after 24 hours, discard the culture medium, wash once with sterilized PBS, add ROS staining solution DCFH (10 μM), mitochondrial membrane potential staining solution JC-1 (2.5 μg / mL) or apoptosis staining solution PI / FITC (2:1), and incubate in the dark in th...
Embodiment 3
[0077] The immunofluorescence technique involved in this embodiment is divided into negative control group (without adding any drugs), model group (H-Glu+Insulin), coumarin group (H-Glu+Insulin+7-Hyd) and comparison drugs Group (H-Glu+Insulin+Irb). The final concentration of irbesartan is 13.5mg / mL, and the final concentration of coumarin is 0.5mg / mL.
[0078] The main steps include:
[0079] 1. Preparation of cell suspension: trypsinize GEnCs cells derived from kidney, count and resuspend cells in complete medium.
[0080] 2. Cell slides: Add a small amount of complete medium dropwise to each well of the six-well plate, so that the coverslip and the six-well plate can be bonded together by the tension of the complete medium, and then put the cleaned and sterilized coverslip. The cells were evenly inserted into six-well plates covered with 18mm×18mm coverslips. Put it into the cell incubator, add 2mL complete medium to each well after about 1h-2h when 80% of the cells adher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com