Preparation method of choline receptor antagonist
A choline receptor and antagonist technology, applied in the field of drug synthesis, can solve the problems of high cost, low yield, unsuitable for industrial production, etc., and achieves high reaction yield and product purity, simple operation, and remarkable economic benefits. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
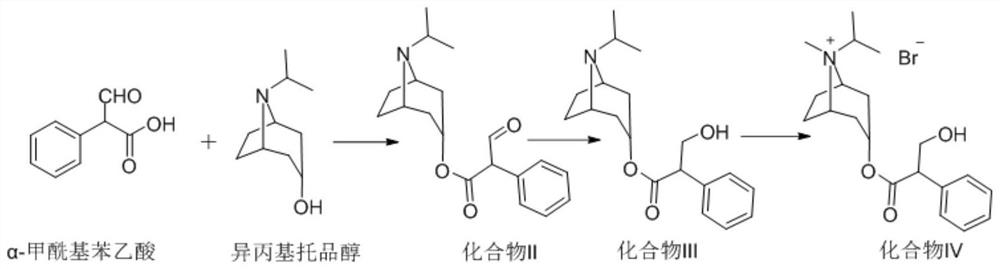
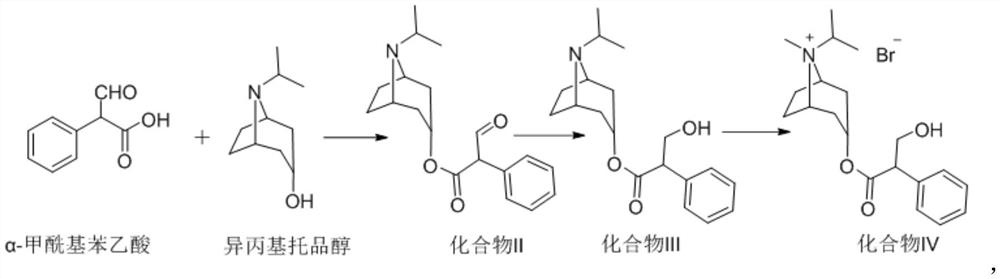
[0046] Step 1): Preparation of 2-formylphenylacetic acid-(1R,3r,5S)-8-isopropyl-8-nitrobicyclo[3.2.1]octane-3-yl ester (compound II)
[0047] Under nitrogen protection, 164.27g of α-formylphenylacetic acid (1.0mol) and 1L of tetrahydrofuran were added to the reaction flask, the temperature was lowered to 0-5°C, and 243.23g (1.5mol) of N,N'-carbonyldiimidazole was added. Keep stirring at 0-5° C. for 1 hour, and then add 186.20 g (1.1 mol) of isopropyltropine alcohol. After the addition is complete, keep the reaction at 20-30°C for 8 hours. Then lower the temperature to 0-5°C, add 1L water in drops while keeping warm, keep stirring at 0-5°C for 2 hours after dropping the water, filter, and vacuum-dry the filter cake at 60°C until constant weight to obtain 299.64g off-white solid. Yield 95%, purity HPLC: 99.8%;
[0048]1HNMR (DMSO-d6): δppm 1.05-1.08 (d, 6H), 1.54-1.58 (d, 2H), 1.72-1.74 (d, 4H), 2.05-2.09 (m, 2H), 2.77-2.80 (m, 1H), 3.36-3.58(m, 3H), 4.96-4.98(t, 1H), 7.09-7....
Embodiment 2
[0062] Step 1): Preparation of 2-formylphenylacetic acid-(1R,3r,5S)-8-isopropyl-8-nitrobicyclo[3.2.1]octane-3-yl ester (compound II)
[0063] Under nitrogen protection, 164.27g of α-formylphenylacetic acid (1.0mol) and 1L of tetrahydrofuran were added to the reaction flask, the temperature was lowered to 0-5°C, and 162.15g (1.0mol) of N,N'-carbonyldiimidazole was added. Keep stirring at 0-5° C. for 1 hour, and then add 186.20 g (1.1 mol) of isopropyltropine alcohol. After the addition is complete, keep the reaction at 20-30°C for 8 hours. Then lower the temperature to 0-5°C, drop 1L of water into it while keeping it warm, keep stirring at 0-5°C for 2 hours after dropping the water, filter, and vacuum-dry the filter cake at 60°C until constant weight to obtain 293.33g of off-white solid. Yield 93%, purity HPLC: 99.6%. NMR results are the same as in Example 1.
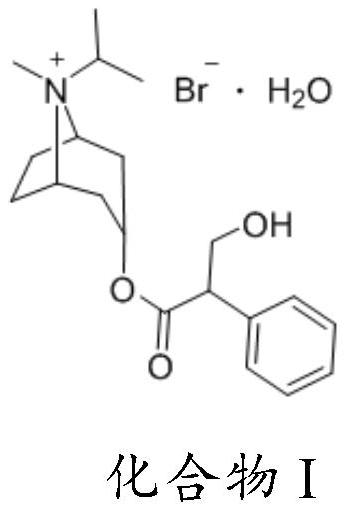
[0064] Steps 2), 3), and 4) are consistent with Example 1.
Embodiment 3
[0066] Step 1): Preparation of 2-formylphenylacetic acid-(1R,3r,5S)-8-isopropyl-8-nitrobicyclo[3.2.1]octane-3-yl ester (compound II)
[0067] Under nitrogen protection, 164.27g of α-formylphenylacetic acid (1.0mol) and 1L of tetrahydrofuran were added to the reaction flask, the temperature was lowered to 0-5°C, and 486.45g (3.0mol) of N,N'-carbonyldiimidazole was added. Keep stirring at 0-5° C. for 1 hour, and then add 186.20 g (1.1 mol) of isopropyltropine alcohol. After the addition is complete, keep the reaction at 20-30°C for 8 hours. Then lower the temperature to 0-5°C, drop 1L of water into it while keeping it warm, keep stirring at 0-5°C for 2 hours after dropping the water, filter, and vacuum-dry the filter cake at 60°C to constant weight to obtain 290.18g of off-white solid. Yield 92%, purity HPLC: 99.7%. NMR results are the same as in Example 1.
[0068] Steps 2), 3), and 4) are consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com