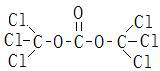Preparation method of environment-friendly fomesafen
A kind of fomesafen, environment-friendly technology, applied in the field of synthesis and preparation of fomesafen, can solve problems such as pollution, achieve the effects of improving conversion speed, easy transportation and storage, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
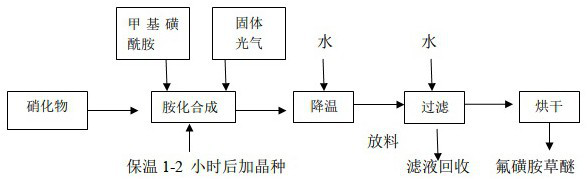
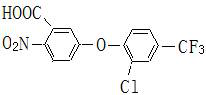
[0041] refer to figure 1 , using nitrate 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid, methylsulfonamide and solid phosgene to synthesize fluorosulfonamide under the action of dichloroethane grass ether. 5-[2-Chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid dichloroethane was extracted and then transferred to the reaction kettle, 5-[2-chloro-4-(trifluoromethyl) The input amount of methyl)phenoxyl]-2-nitrobenzoic acid is 383 parts, and after dichloroethane is qualified with water, the input amount of methylsulfonamide is 114 parts, and solid light is added at a uniform speed, and the input of solid phosgene The amount is 118 parts, the temperature is kept below 90°C, after the addition of solid light, keep it warm for 2 hours, add the seed crystal and continue the reaction at 100-120°C, the reaction time is 12h, then lower the temperature, add water, filter, and dry to get fluorosulfonamide Finished product of grass ether. The mass fraction of the finish...
Embodiment 2
[0044] refer to figure 1 , using nitrate 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid, methylsulfonamide and solid phosgene to synthesize fluorosulfonamide under the action of dichloroethane grass ether. 5-[2-Chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid dichloroethane was extracted and then transferred to the reaction kettle, 5-[2-chloro-4-(trifluoromethyl) The input amount of methyl)phenoxyl]-2-nitrobenzoic acid is 383 parts, and after dichloroethane is qualified with water, the input amount of methylsulfonamide is 104 parts, and solid light is added at a uniform speed, and the input of solid phosgene The amount is 109 parts, the temperature is kept below 90°C, after the addition of solid light, keep it warm for 2 hours, add seed crystals and continue the reaction at 100-120°C, the reaction time is 12h, then lower the temperature, add water, filter, and dry to obtain fluorosulfonamide Finished product of grass ether. The mass fraction of the finish...
Embodiment 3
[0047] refer to figure 1, using nitrate 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid, methylsulfonamide and solid phosgene to synthesize fluorosulfonamide under the action of dichloroethane grass ether. 5-[2-Chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid dichloroethane was extracted and then transferred to the reaction kettle, 5-[2-chloro-4-(trifluoromethyl) The input amount of methyl)phenoxyl]-2-nitrobenzoic acid is 383 parts, and after dichloroethane is qualified with water, the input amount of methylsulfonamide is 114 parts, and solid light is added at a uniform speed, and the input of solid phosgene The amount is 118 parts, the temperature is kept below 90 ° C, after the addition of solid light, keep it warm for 2 hours, add 8 parts of catalyst to the reaction at 100-120 ° C, remove the catalyst after 8 hours of reaction, continue to react for a total time of 10 hours, then lower the temperature and add water , filtered, and dried to obtain the fin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com