Synthetic method of L-nicotine with optical activity
A synthetic method and optically active technology, applied in the fields of organic chemistry, organic chemistry, etc., can solve the problems of high cost of biological enzyme system, long enzyme catalytic reaction time, narrow operating range, etc., and achieve a small amount of catalyst, short reaction time, stable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
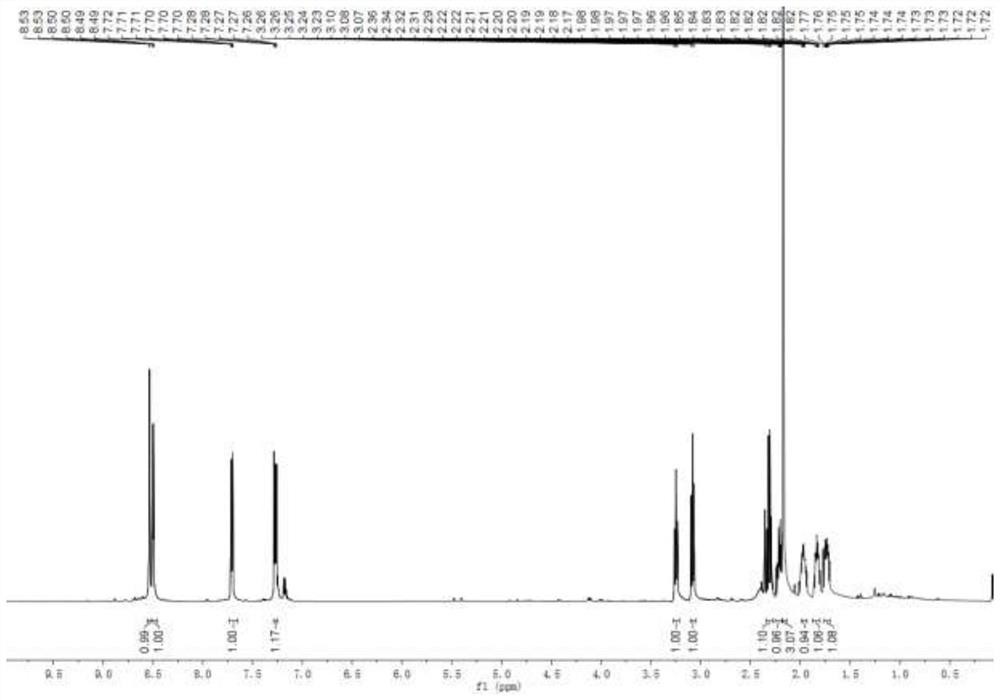
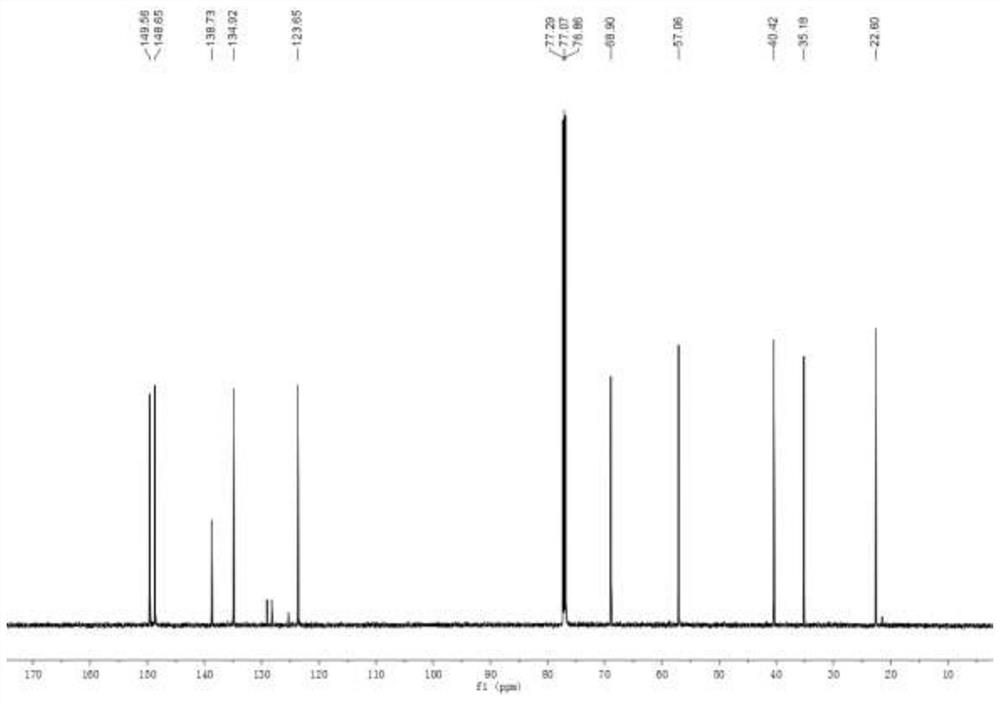
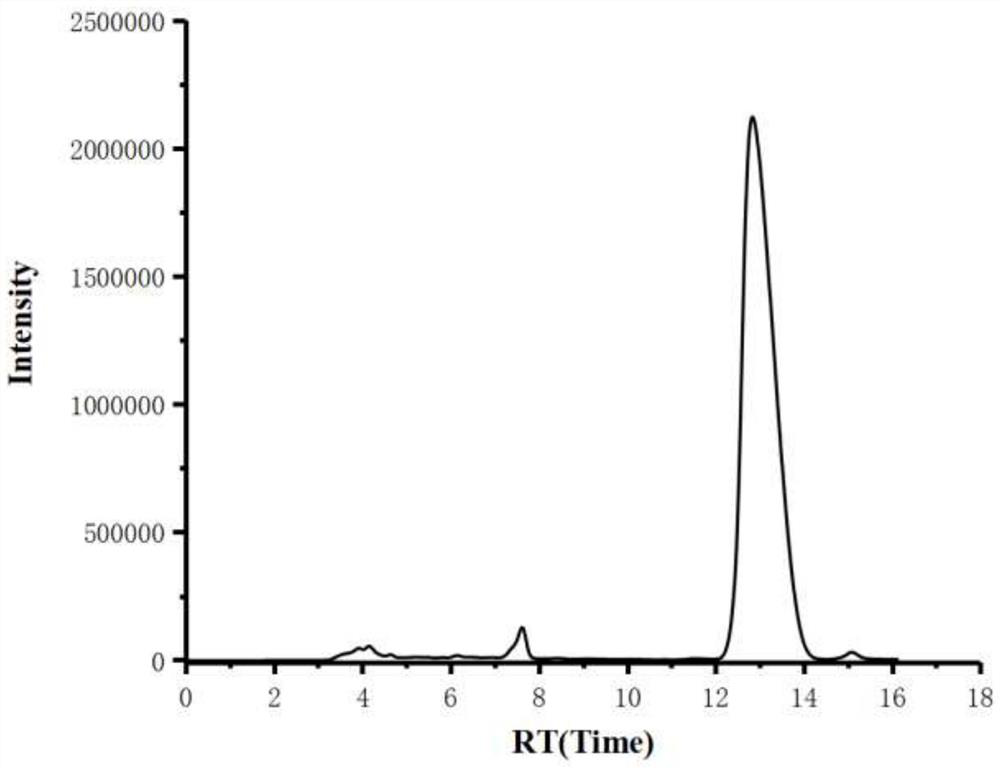
[0030] Add mesmin (0.5mmol) and iridium-phosphoroxazoline catalyst (0.005mmol) in the Schlenk tube protected by nitrogen, replace the nitrogen in the Schlenk tube with hydrogen, add 4ml of 1,2-dichloroethane, and Under reaction for 24 hours. After spin-drying the solvent, 5 ml of toluene, formic acid (1 mmol), and paraformaldehyde (0.6 mmol) were added, and stirred at 65° C. for 3 hours. After the reaction was complete, aqueous NaOH solution was added to adjust the pH of the mixture to 13, and water was added until the solid dissolved. Extract three times with ethyl acetate, combine organic phase, dry, rotary evaporate to obtain crude product, obtain the L-nicotine (99%ee) of light yellow oily liquid with the productive rate of 68% with silica gel column chromatography, product H NMR spectrum Such as figure 1 As shown, the carbon NMR spectrum is as figure 2 As shown, the chromatogram is shown as image 3 shown. 1 H NMR (600MHz, CDCl 3 ), δ=8.53(d, J=1.9Hz, 1H), 8.50(dd,...
Embodiment 2
[0032] Add mesmin (1.0mmol) and iridium-phosphoroxazoline catalyst (0.01mmol) into the Schlenk tube protected by nitrogen, replace the nitrogen in the Schlenk tube with hydrogen, add 7ml of 1,2-dichloroethane, and Under reaction for 24 hours. After spin-drying the solvent, 10 ml of toluene, formic acid (2 mmol), and paraformaldehyde (1.2 mmol) were added, and stirred at 65° C. for 3 hours. After the reaction was complete, aqueous NaOH solution was added to adjust the pH of the mixture to 12, and water was added until the solid dissolved. Extracted three times with ethyl acetate, combined the organic phases, dried, and rotary evaporated to obtain the crude product, and obtained the light yellow oily liquid L-nicotine (98.5% ee) with a yield of 65% by silica gel column chromatography.
Embodiment 3
[0034] Add mesmin (1.5mmol) and iridium-phosphoroxazoline catalyst (0.015mmol) into the Schlenk tube protected by nitrogen, replace the nitrogen in the Schlenk tube with hydrogen, add 10ml of 1,2-dichloroethane, and Under reaction for 24 hours. After spin-drying the solvent, 10 ml of toluene, formic acid (3 mmol), and paraformaldehyde (1.8 mmol) were added, and stirred at 65° C. for 3 hours. After the reaction was complete, aqueous NaOH solution was added to adjust the pH of the mixture to 13.5, and water was added until the solid dissolved. Extracted three times with ethyl acetate, combined the organic phases, dried, and rotary evaporated to obtain the crude product, and obtained light yellow oily liquid L-nicotine (97% ee) with a yield of 64% by silica gel column chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com