Method for absorbing and refining high-purity methylacrolein
A methacrolein and refining method technology, applied in the field of absorption and refining of high-purity methacrolein, can solve the problems of high loss of methanol, low absorption efficiency, difficult separation, etc., and achieve less loss of absorbent and less loss of absorbent Small size, high process safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
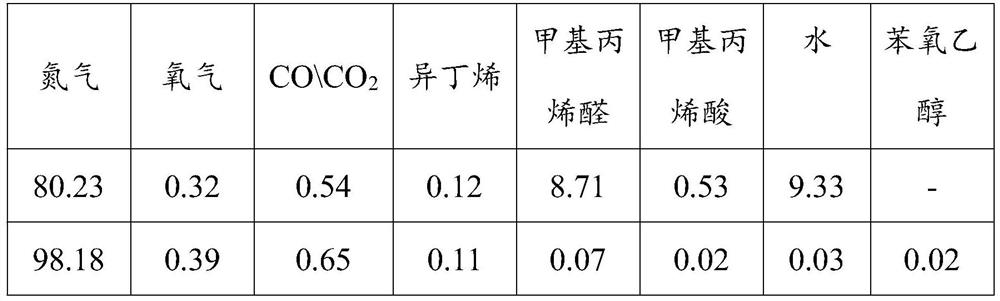
[0032] The gas phase product after isobutene oxidation is cooled to 15°C and enters the packed absorption tower. Use 5 times the gas mass of phenoxyethanol (15°C) for absorption. The volume content of the gas before and after absorption is shown in Table 1 (the data in the table are volume percentage content).
[0033]
[0034] The content of methacrolein in the heavy phase obtained from the absorption liquid obtained at the bottom of the absorption tower after standing still for 1 hour and separated was 6.4%. In the rectification operation, the temperature at the bottom of the tower was 75° C., and the reflux ratio was 0.5 to obtain the high-purity product methacrolein, the composition of which is shown in Table 2 (the data in the table are percentages by mass).
[0035]
[0036]
Embodiment 2
[0038] The gas phase product after isobutene oxidation is cooled to 15°C and enters the packed absorption tower. Diethylene glycol monophenyl ether (15°C) 5 times the mass of the gas is used for absorption. The volume content of the gas before and after absorption is shown in Table 3 (the data in the table are volume percentage content).
[0039]
[0040] The content of methacrolein in the heavy phase obtained from the absorption liquid obtained at the bottom of the absorption tower after standing for 2 hours and stratified was 6.1%, and the heavy phase was subjected to rectification. In the rectification operation, under the condition that the tower bottom temperature is 80° C. and the reflux ratio is 1.0, a high-purity product methacrolein is obtained, and its composition is shown in Table 4 (the data in the table are mass percentages).
[0041] methacrolein Methacrylate Acetic acid water other 99.43 0.14 0.06 0.20 0.18
Embodiment 3
[0043] The gas phase product after isobutene oxidation is cooled to 15°C and enters the packed absorption tower. A mixture of phenoxyethanol and diethylene glycol monophenyl ether 5 times the mass of the gas is used for absorption. In the mixture, (the mass percent content of phenoxyethanol is 90%, and the temperature is 15° C.). The volume content of the gas before and after absorption is shown in Table 5 (the data in the table are volume percentage content).
[0044]
[0045] The content of methacrolein in the absorption liquid obtained at the bottom of the absorption tower was 6.3%. After standing still for 1.5 hours, the heavy phase was obtained by stratification, and the heavy phase was subjected to rectification. In the rectification operation, under the condition that the tower bottom temperature is 80° C. and the reflux ratio is 0.5, a high-purity product methacrolein is obtained, and its composition is shown in Table 6 (the data in the table are percentages by mass...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap