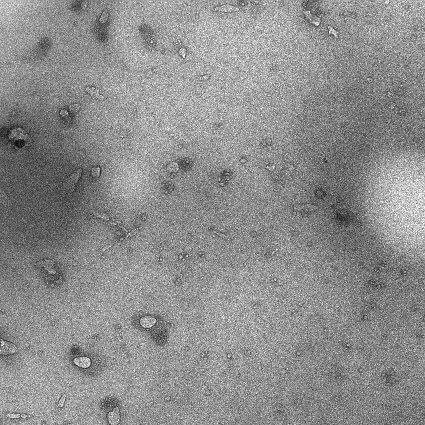
Hydrogel containing doxorubicin and immunologic adjuvant combined drug liposome for injection and preparation method thereof
A technology of immune adjuvant and water for injection, which is applied in the field of hydrogel for injection containing liposome combined with doxorubicin and immune adjuvant and its preparation field, can solve the problem of insufficient anti-tumor effect, difficulty in inhibiting tumor cell growth, and resistance to tumor cells. medicine and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: prepare the PLGA-PEG-PLGA (polyglycolide-lactide-polyethylene glycol-polyglycolide lactide) hydrogel containing doxorubicin and CpG co-loaded liposomes, referred to as ( ADR / CpG-Lip) PLGA-Gel
[0048] Preparation prescription (50g quantitative):
[0049] Doxorubicin 100mg
[0050] CpG 10mg
[0051] Distearic Lecithin (DSPC) 640mg
[0052] DOTAP 140mg
[0053] PLGA-PEG-PLGA block copolymer 6g (PLGA(75 / 25)1870-PEG1500-PLAG(75 / 25)1870 or PLGA(75 / 25)1704-PEG1500-PLAG(75 / 25)1704)
[0054] Cholesterol 190mg
[0055] Vitamin E 2.4mg
[0056] Sodium dihydrogen phosphate 160mg
[0057] Sucrose 1g
[0058] Quantitative water for injection to 50g
[0059] The preparation process is as follows:
[0060] According to the formula, DSPC, DOTAP, cholesterol and vitamin E were mixed in 4% ethanol solution and heated to 65°C to form a lipid solution; another ammonium sulfate with a concentration of 250mM was prepared to dissolve doxorubicin and CpG and heated to 6...
Embodiment 2
[0061] Example 2: Preparation of PCL-PEG-PCL (polycaprolactone-polyethylene glycol-polycaprolactone) hydrogel containing doxorubicin and PolyICLC co-loaded liposomes, referred to as (ADR / PolyICLC-Lip) PCL-Gel
[0062] Preparation prescription (50g quantitative):
[0063] Doxorubicin 100mg
[0064] PolyICLC 20mg
[0065] Hydrogenated Soy Lecithin (HSPC) 760mg
[0066] Dlin-MC3-DMA 220mg
[0067] Cholesterol 220mg
[0068] PCL-PEG-PCL 6.4g (PCL2000-PEG1500-PCL2000 or PCL3400-PEG1500-PCL3400)
[0069] Vitamin E 2.0mg
[0070] Sucrose 2g
[0071] Sodium dihydrogen phosphate 240mg
[0072] Quantitative water for injection to 50g
[0073] The preparation process is as follows:
[0074] According to the recipe, HSPC, Dlin-MC3-DMA, cholesterol and vitamin E were mixed in 4% ethanol solution and heated to 55°C to form a lipid solution; another ammonium sulfate with a concentration of 250mM was prepared to dissolve doxorubicin and PolyICLC and heated to 60 ℃; and the lipid so...
Embodiment 3
[0075] Example 3: Preparation of PDLLA-PEG-PDLLA (poly D, L-lactide-polyethylene glycol-poly D, L-lactide) hydrogel containing doxorubicin and lipopolysaccharide co-loaded liposomes , referred to as (ADR / LPS-Lip) PDLLA-Gel
[0076] Preparation prescription (50g quantitative):
[0077] Doxorubicin 100mg
[0078] Lipopolysaccharide 35mg
[0079] Distearate Phosphatidylglycerol (DSPG) 160mg
[0080] Egg Yolk Phosphatidylglycerol (EPG) 240mg
[0081] c12-200 220mg
[0082] Cholesterol 220mg
[0083] Vitamin E 1.6mg
[0084] PDLLA(1500-2000)-PEG(1000-1500)-PDLLA(1500-2000) 8.5g
[0085] Sodium dihydrogen phosphate 200mg
[0086] Dilute to the required volume with water for injection
[0087] The preparation process is as follows:
[0088] According to the formula, DSPG, EPG, c12-200, cholesterol and vitamin E were mixed in 4% ethanol solution and heated to 58°C to form a lipid solution; another ammonium sulfate with a concentration of 250mM was prepared to dissolve doxoru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com