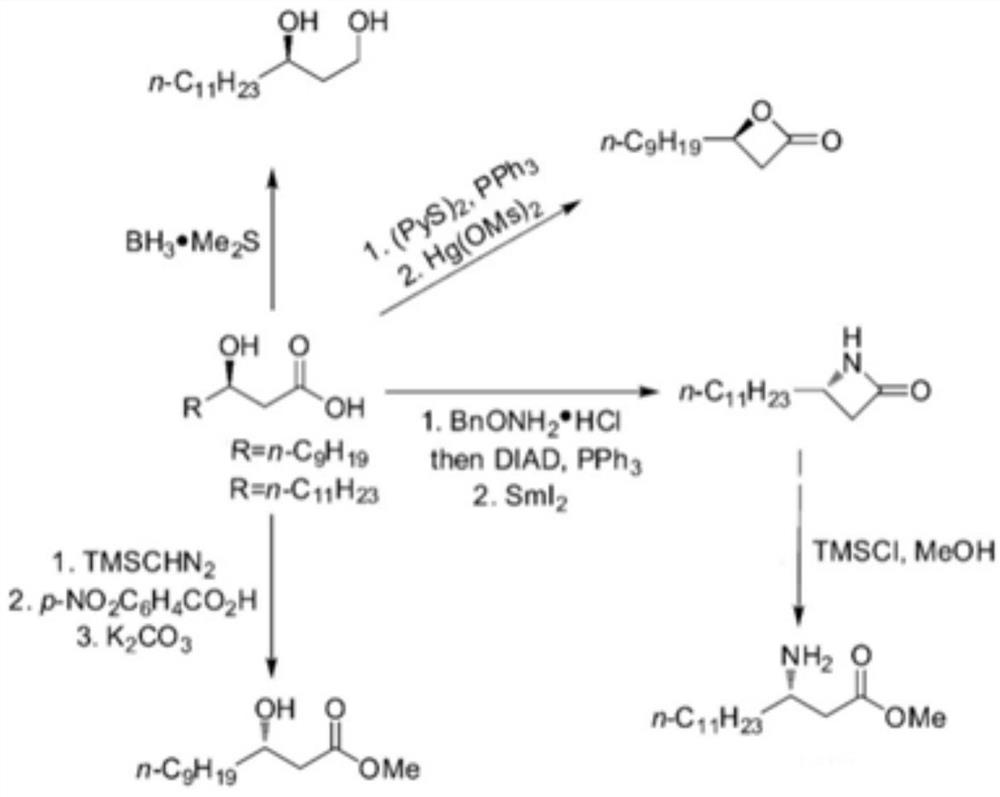
Artificial biocatalyst for converting fatty acid into beta-hydroxy fatty acid
A technology of hydroxy fatty acid and fatty acid, which is applied in the field of protein engineering to achieve the effect of simple reaction system, low price and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1P450
[0046] The construction of embodiment 1P450BSβ gene expression vector
[0047] The P450BSβ fragment synthesized from the whole gene (sequence shown in SEQ ID NO: 3, synthesized by Suzhou Jinweizhi Biological Co., Ltd.) was double-digested with restriction endonucleases NcoI and XhoI (New England Biolabs) according to the instructions , T4 ligase (New England Biolabs) was used to connect to the expression vector pET28a cut by NcoI and XhoI. The ligation product was transformed into Escherichia coli DH5α competent cells (Tiangen Biochemical Technology Co., Ltd.). Pick the successfully transformed monoclonal colony from the solid LB medium plate containing 50 μg / ml kanamycin, and place it in the LB liquid medium containing the same concentration of kanamycin at 37°C with a shaker speed of 220rpm / min conditions overnight. The recombinant plasmid was extracted from the cultured overnight bacterial solution using a small plasmid extraction kit (Tiangen Biochemical Technology Co., ...
Embodiment 2P450
[0048] Large amount of expression and purification of embodiment 2P450BSβ mutant protein
[0049] Pick glycerol bacteria stored in a -80°C ultra-low temperature refrigerator and inoculate into 40ml TB liquid medium (Kan / Cam) for overnight culture at 37°C. Inoculate 500ml of TB medium (Kan / Cam) with the overnight cultured bacterial solution, and carry out expansion culture at 37°C with a shaker speed of 220rpm / min; 600 At about 0.6, add precursor ALA (δ-aminolevulinic acid) with a final concentration of 0.5mM and 1mM IPTG to induce expression, and culture at 25°C with a shaker speed of 220rpm / min for 16h. Centrifuge at 5000rpm / min for 10 min, collect the bacteria, and resuspend the bacteria with 75ml of buffer A (0.1M KPi, 0.3M KCl, 20% glycerol, pH 7.4), and then use ultrasound to disrupt. Centrifuge at 10000 rpm / min at 4°C for 40 min to obtain the supernatant. Put the supernatant containing the target protein into the prepacked nickel ion affinity column to bind the target ...
Embodiment 3
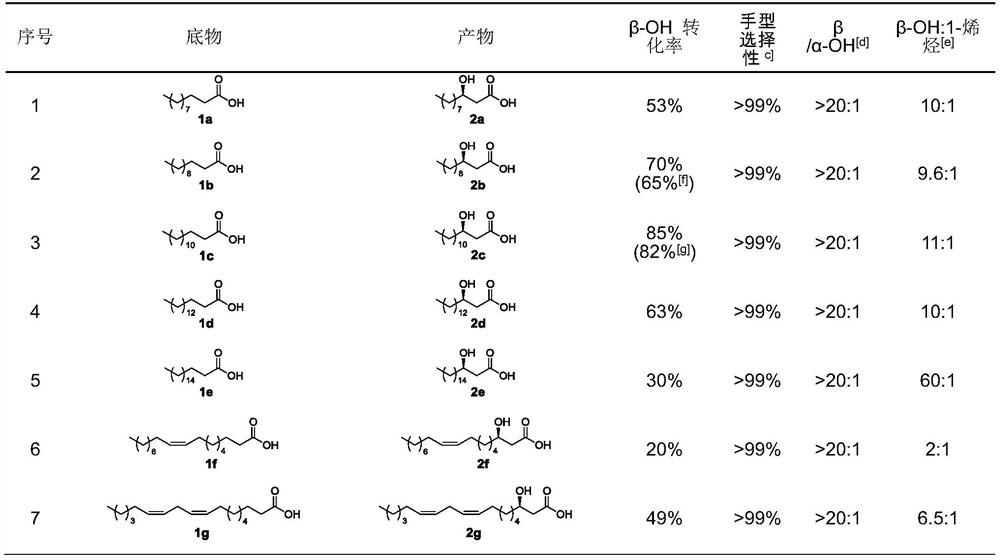
[0050] Example 3 β-hydroxylase P450BSβ mutant catalyzes the generation of β-hydroxy fatty acids
[0051] Fatty acid β-hydroxylation reaction contains bufferA, purified β-hydroxylase P450BSβ mutant (4 μM), substrate (4 mM), Triton X-100 (0.5%), 5% ethanol as co-solvent, hydrogen peroxide (5 mM ×2, interval time=5min). The reaction was carried out in a final volume of 100 mL for 45 min at room temperature. A large-scale reaction for the hydroxylation of dodecanoic acid (200mg, 1.0mmol) and tetradecanoic acid (570mg, 2.50mmol) was carried out under the same conditions, except that the substrate concentration was 5mM, the enzyme was 3uM, Triton X- 100 (0.625%). After HCl quenching, 1 mL of the reaction mixture was removed, extracted, treated with TMSCHN2, and analyzed by GC and GC-MS. The remaining reaction solution was extracted with ethyl acetate and washed with anhydrous MgSO 4 Dry and filter. The filtrate was concentrated in vacuo and purified by silica gel column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com