Preparation and application of anthraquinone artificial hapten, immunogen, artificial coating antigen and antibody
An artificial hapten and hapten technology, applied in the preparation of organic compounds, immunoglobulin, carboxylic acid amide preparation, etc., to achieve the effect of simple and fast operation, high similarity and good detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
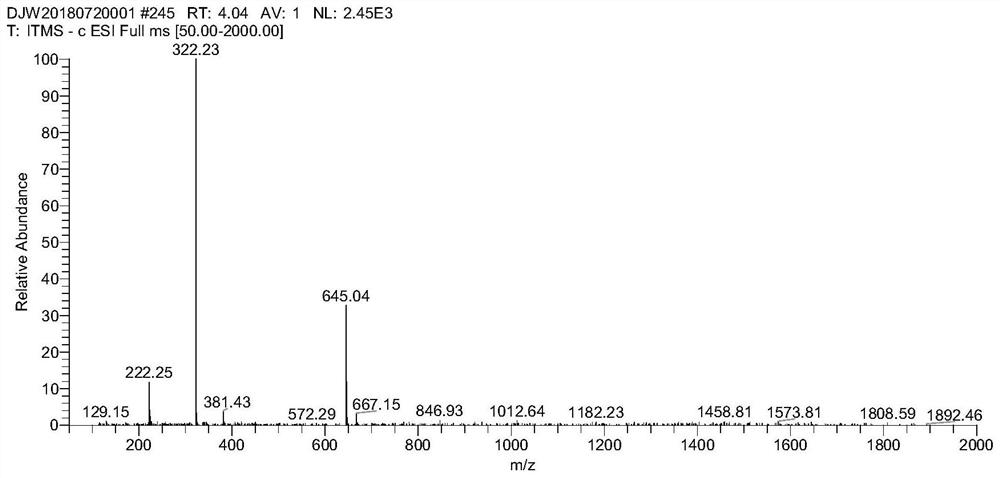
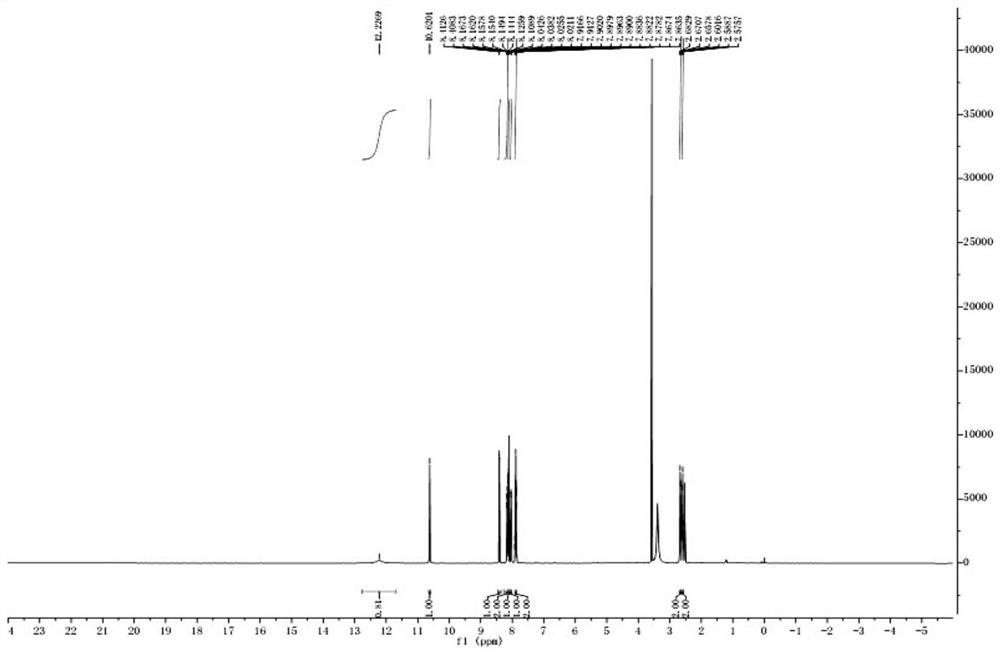
[0056] (1) Synthesis of anthraquinone hapten
[0057] Weigh 2.23g (0.01mol) of 2-aminoanthraquinone into a 100ml three-neck flask, add 20ml of glacial acetic acid, heat up to 65°C and stir to dissolve, then weigh 1.1g (0.011mol) of succinic anhydride and dissolve it in 3mL of glacial acetic acid In the three-necked flask, it was added dropwise to keep warm for 6 hours. Cool down to 5-10°C, filter with suction, dry the obtained solid, and recrystallize with 1,4-dioxane. After recrystallization, a yellow solid was precipitated, and after filtration and drying, 1.62 g of a yellow solid was obtained, with a yield of 50.1%. 1 H NMR (500MHz, DMSO-d6), δ (ppm): 12.22 (s, 1H), 10.62 (s, 1H), 8.41 (d, J = 2.15, 1H), 8.17-8.14 (m, 2H), 8.12 (d, J=8.5,1H),8.03(dd,J=2.2,2.2,1H),7.92-7.86(m,2H),2.67(t,J=6.1,6.45,2H),2.59(t,J =6.45,6.5,2H).
[0058] (2) Preparation of anthraquinone immunogen and coating original
[0059] Dissolve 0.01mmoL anthraquinone hapten in 1mL DMF, add 0.2mL DMF ...
Embodiment 2
[0065] The anthraquinone monoclonal antibody obtained in Example 1 was used for immunodetection.
[0066] (1) Coating: Dissolve anthraquinone-OVA in 0.05mol / L carbonic acid buffer (pH 9.6), coat 100μl / well on a microplate, and coat overnight at 4°C in a refrigerated environment. After taking it out, use 0.01M Wash 3 times with PBST.
[0067] (2) Sealing: After the coating is completed, prepare 2% skimmed milk powder with 0.01mol / L PBS (pH 7.4), seal the microplate with 300μl / well, and seal it in a 37°C incubator for 30 minutes. After taking it out, use 0.01mol / L Wash 2 times with L PBST.
[0068] (3) Competitive reaction: dissolve the sample to be tested in 0.01mol / L PBS solution of 30% methanol, add 50 μl / well to the microtiter plate, then add 50 μl / well of anthraquinone antibody solution, and react in a 37°C incubator for 1 hour , wash the plate 3 times after taking it out.
[0069] (4) Enzyme-labeled secondary antibody reaction: Dilute rabbit anti-mouse enzyme-labeled se...
Embodiment 3
[0074] Cross-reactivity assay was performed using the anthraquinone antibody obtained in Example 1.
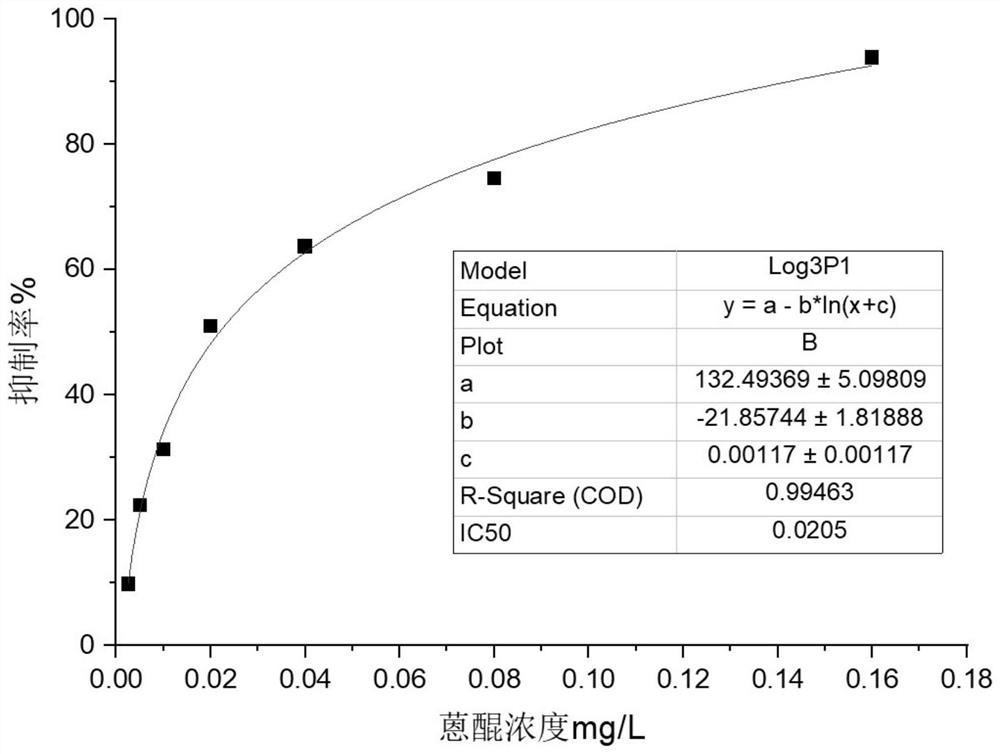
[0075] Use the checkerboard test method to determine that the coating concentration of the coating source is 5 μg / L, the coating volume is 100 μL per well, the antibody reaction concentration is 1 μg / L, and the dilution factor of the rabbit anti-mouse enzyme-labeled secondary antibody is 1:40000. Antibody specificity was determined below. According to the degree of cross-reaction between antibody and anthraquinone compound, to inhibit antibody 50% (IC 50 ) Concentration of desired target analyte and approximate target analyte IC 50 Expressed as a percentage of the concentration ratio, that is, the cross-reactivity rate C.R (%). C.R(%)=S=Y / Z×100%, Y: IC 50 Concentration of target analyte at time, Z: IC 50 approximate the concentration of the target analyte. The smaller the cross-reaction rate, the higher the specificity of the antibody; the larger the cross-reaction rate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com