Preparation method of CAR-T cell with CD133 specificity, based on non-viral vector and capable of automatically secreting PD1 scFv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Construction of minicircle CAR plasmid:
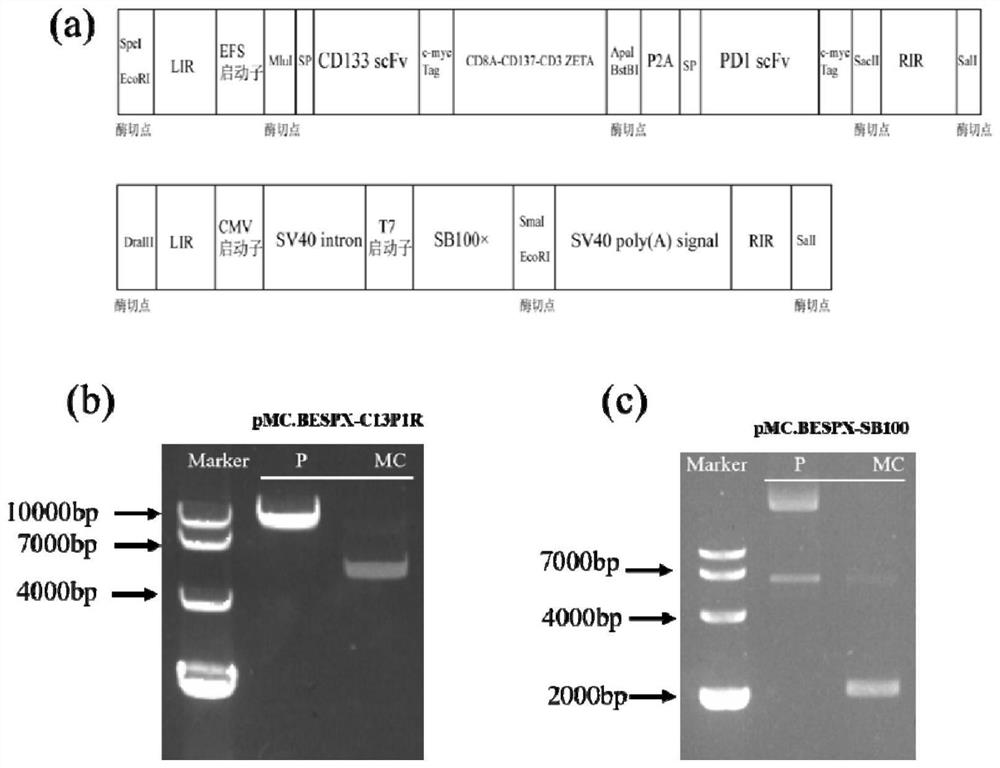
[0032] By searching the CD133 antibody and PD1 antibody sequences, the scFv segments in the CD133 and PD1 antibody sequences were selected, and the second-generation CAR-T technology was used to design the CAR-T sequence, and the PD1 scFv was connected through P2A. The terminal repeat sequence required by the seat system is constructed by the company and verified by sequencing.
[0033] Construct the above sequence into the multifunctional cloning site in the parent plasmid pMC.BESPX-MCS for preparing the minicircle; purchase the transposase plasmid pCMV(CAT)-T7-SB100, and CMV-SB100×-SV40 polyA on the plasmid The segment was integrated into the minicircle parent plasmid pMC.BESPX-MCS through homologous recombination; the above two plasmids were sequenced and verified (see figure 1 Figure a).
[0034] The above plasmid was transformed into competent cells ZYCY10P3S2T by heat shock method, then 200 μl of SOC medium wa...
Embodiment 2
[0035] Example 2: CAR-T cell preparation stage
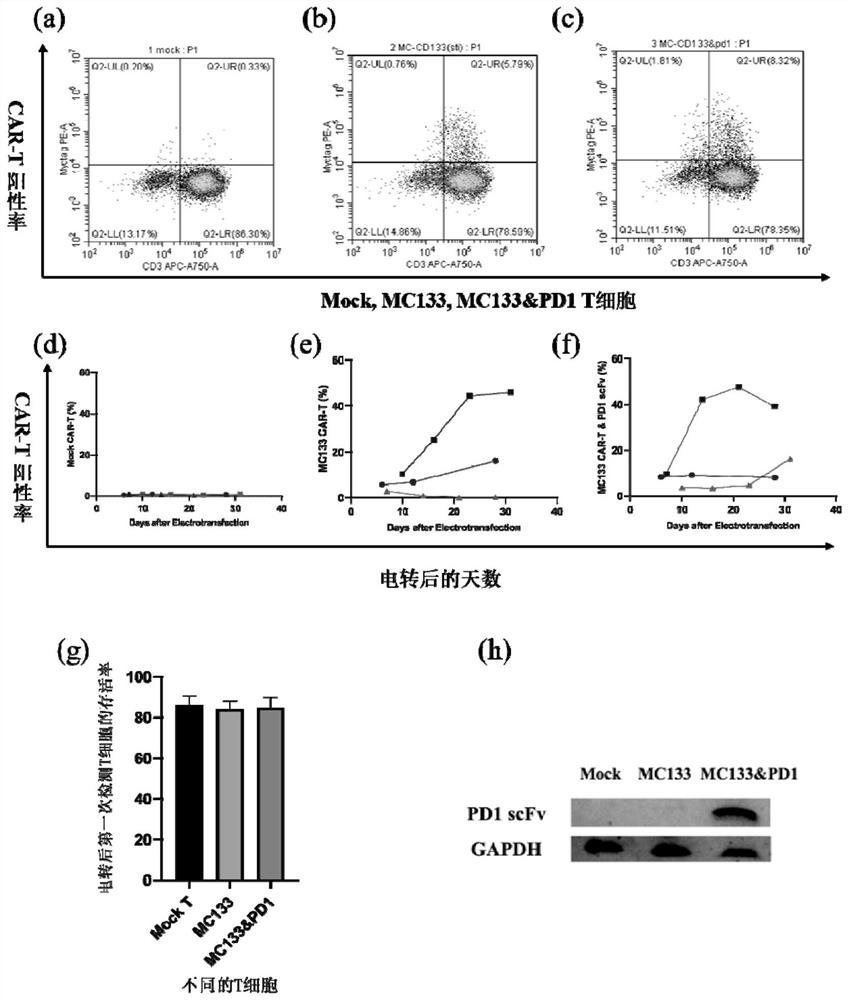
[0036] Take blood from a healthy donor, centrifuge at 500g for 10min, discard the upper plasma, add an equal volume of PBS, resuspend, transfer to a 50ml centrifuge tube reserved for 20ml of lymphocyte separation medium, make up to 50ml with normal saline; centrifuge at 800g for 20min (liter 9, down 6), divided into 4 layers, take the white floc (white film) into a new 15ml centrifuge tube, wash the white film with elution buffer, count; every 10 7 For each T cell, add 5ul Pan T Cell Biotin antibody to 60μl elution buffer, incubate at 4°C for 5min; then add 10ul Pan T cell magnetic beads and incubate at 4°C for 10min; after the incubation, add the liquid to the LS separation column, After sorting by magnetic beads, the cells that come down from the column are T cells; take 1×10 7 Put T cells into a 6-well plate, add 2ml X-vivo, IL-21000IU / ml, and add CD3 / CD28 to stimulate overnight; on the same day, use DPBS to coat CD133 antig...
Embodiment 3
[0041] Example 3: Killing test of CAR-T cells
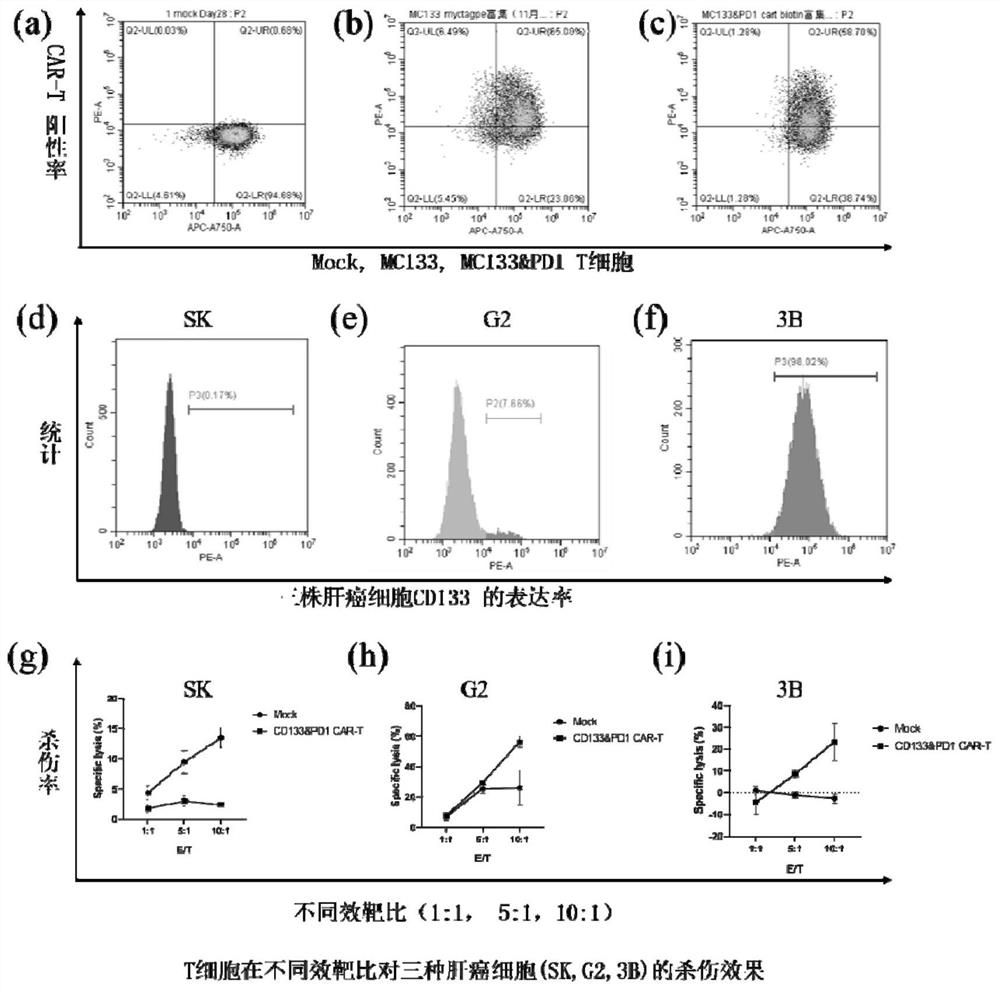
[0042] The liver cancer cell lines SK-Hep1 (SK) and Hep-3B (3B) were selected, and the run-flow analysis showed that there was almost no expression of CD133 on SK, and high expression of CD133 on 3B (>90%). Mock T and MC133&PD1 scFv CAR- After co-incubating T with these two kinds of tumor cells according to the effect-target ratio of 1:1, 5:1, and 10:1 for 4 hours, the apoptosis and death of tumor cells were detected by the apoptosis kit (PI / Annexin-V), and the results It has been shown that the prepared CAR-T cells have stronger killing ability than ordinary T cells (see image 3 ).
[0043] For further verification, the killing ability of MC133 CAR-T alone and MC133&PD1 scFv CAR-T was compared. After co-incubating these two kinds of cells with 3B cells at an effect-to-target ratio of 1:4 for 3 days, it was detected by flow cytometry: MC133&PD1scFv CAR-T cells can almost completely kill tumor cells, while tumor cells still rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com