Recombinant I-type humanized collagen C1L1T and preparation method and application thereof
A technology of collagen and humanization, applied in the field of genetic engineering, can solve the problems of heterologous collagen immune response, such as difficult absorption and utilization, long cycle, low utilization rate of collagen, etc., achieve good cell adhesion effect, and simple preparation method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of recombinant type I humanized collagen C1L1T
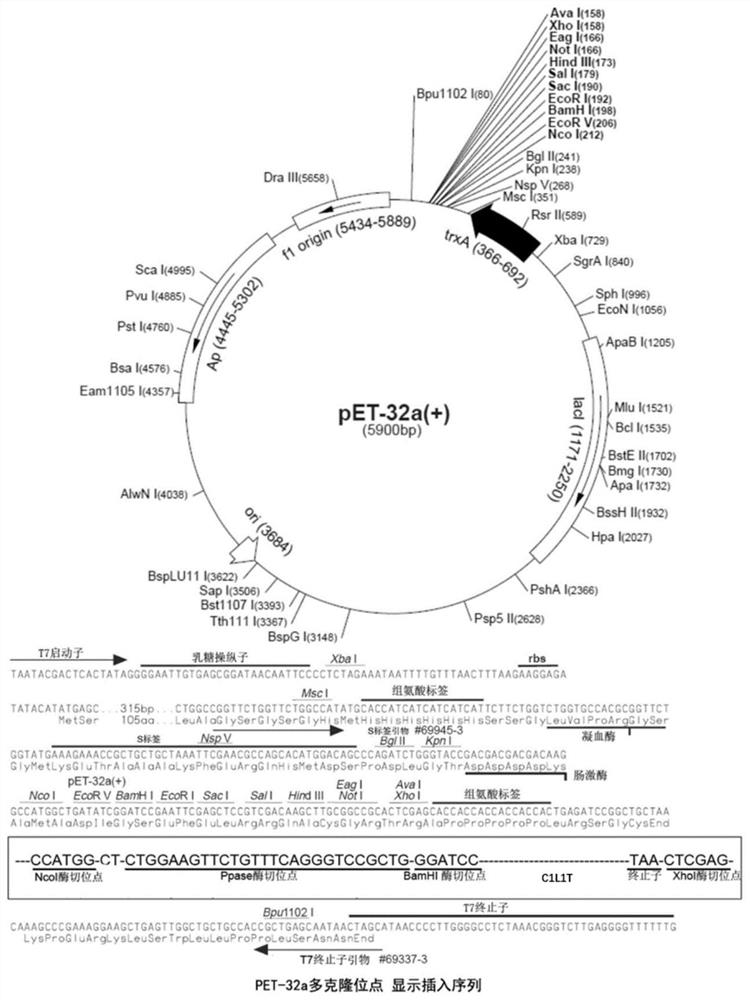
[0055] 1. Construction of C1L1T gene expression vector
[0056] The full-length protein sequence of the recombinant type I humanized collagen C1L1T used in this example is the sequence shown in SEQ ID No.4, with a full-length of 222aa, and the corresponding full-length gene is 666bp. 针对大肠杆菌的密码子进行了密码子优化,优化后的序列为:GGGCCCATGGGACCTTCAGGTCCAAGGGGATTGCCGGGTCCTCCGGGTGCTCCGGGTCCGCAGGGTTTTCAGGGTCCGCCAGGCGAGCCGGGCGAGCCGGGCGCTAGCGGTCCGATGGGTCCGCGTGGTCCGCCGGGCCCGCCGGGCAAGAACGGCGACGACGGTGAGGCAGGCAAACCGGGGCGCCCTGGTGAACGTGGTCCACCGGGTCCGCAAGGTGCGAGAGGCCTGCCCGGTACCGCAGGCCTGCCGGGCATGAAAGGTCACCGTGGCTTCAGCGGTCTGGATGGTGCGAAGGGCGACGCAGGTCCGGCGGGTCCAAAAGGCGAGCCGGGTTCCCCGGGCGAAAATGGCGCGCCTGGCCAGATGGGTCCGCGTGGTTTACCGGGCGAGCGCGGCCGTCCGGGCGCGCCGGGCCCAGCGGGTGCCCGTGGAAACGATGGCGCGACGGGTGCGGCTGGCCCACCGGGCCCGACCGGTCCGGCGGGTCCGCCGGGTTTCCCGGGTGCCGTTGGTGCGAAGGGTGAAGCAGGCCCCCAAGGTCCGCGCGGTTCTGAAGGTCCGCAAGGGGTGCGTGGTGAACCGGGCCCGCCGGGCCCGGC...
Embodiment 2
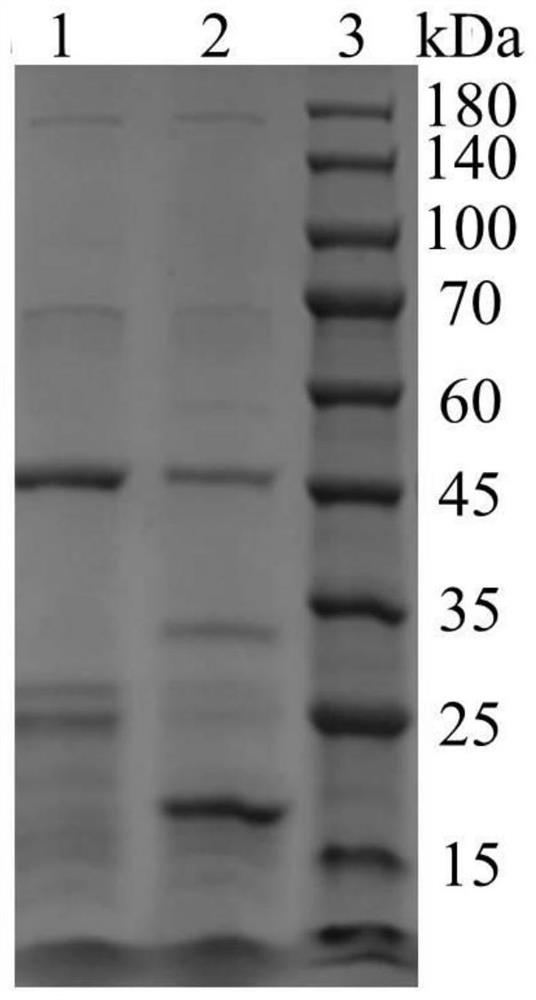
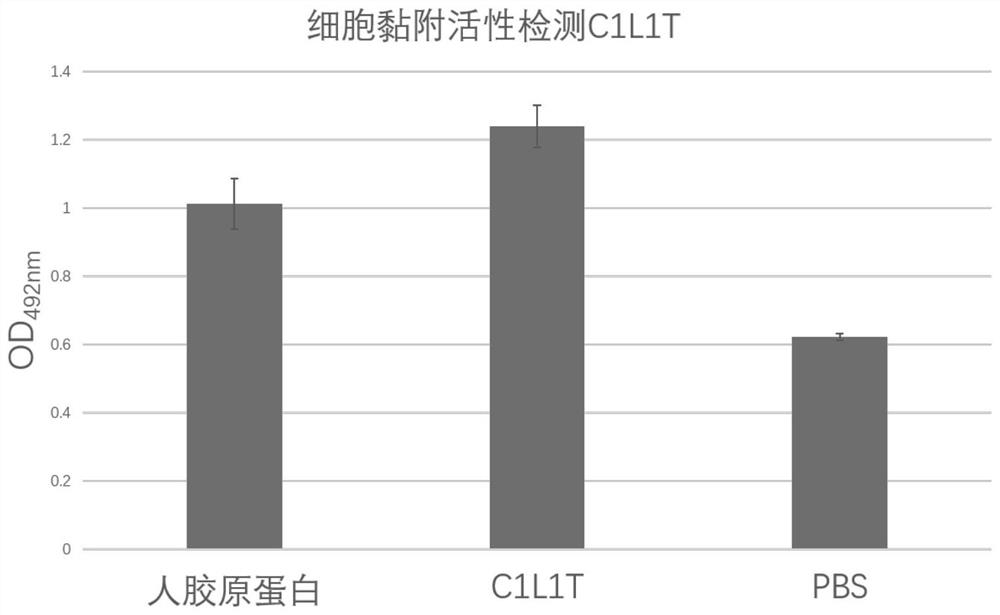
[0068] Example 2: Bioactivity Detection of Recombinant Type I Humanized Collagen C1L1T
[0069] Collagen activity detection method can refer to the literature Juming Yao, Satoshi Yanagisawa, Tetsuo Asakura, Design, Expression and Characterization of Collagen-Like Proteins Based on the Cell Adhesive and Crosslinking Sequences Derived from Native Collagens, J Biochem.136, 643-649 (2004). The specific implementation method is as follows:
[0070] (1) Use the ultraviolet absorption method to detect the concentration of the protein samples to be tested, including commercially available human collagen (Sigma, C7774), and the recombinant type I humanized collagen C1L1T provided by the present invention.
[0071] Specifically, the UV absorption of samples at 215nm and 225nm was measured respectively, and the protein concentration was calculated using the empirical formula C(μg / mL)=144×(A215-A225). Note that the detection should be performed when A215<1.5. The principle of this method...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com