Vaccine vector as well as preparation method and application thereof
A vaccine carrier and vaccine technology, which is applied in the field of biomedicine, can solve the problems of cumbersome and complicated processes and difficulty in large-scale production, and achieve the effect of significant tumor growth, simple and mild preparation conditions, and strong lymph node targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
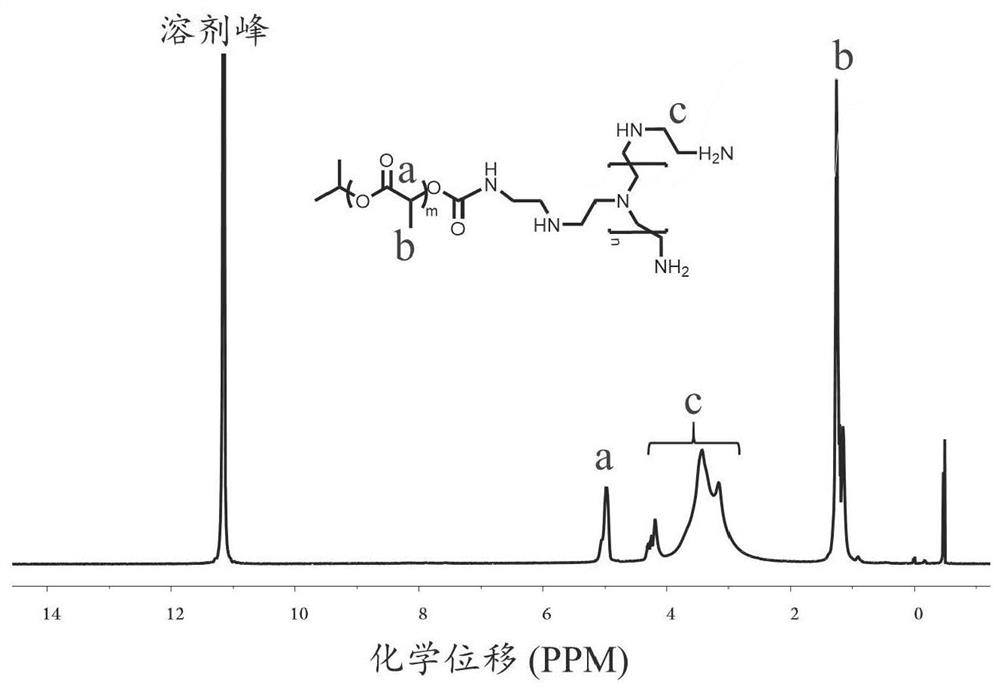
[0045] Preparation of oxidized mannan
[0046] Dissolve 1 g of mannan in 10 mL of sterile water, stir until dissolved in an ice bath, dissolve 657 mg of sodium periodate in 5 mL of sterile water, and add dropwise to the mannan solution. After 2h, the ice bath was removed, and the reaction was carried out at room temperature and protected from light for 12h. The product was purified by dialysis and freeze-dried to obtain the final product.
[0047] The degree of oxidation of the final product was determined by the hydroxylamine hydrochloride method, and the specific operations were as follows: Take 0.1 mg of oxidized mannan, dissolve it in 25 mL of hydroxylamine hydrochloride aqueous solution (0.25mol / L, containing 0.05% methyl orange), and statically Set for 3h. Titrate with standard NaOH solution (0.1mol / L), the red color turns yellow, and the volume of NaOH consumed is recorded as V 1 . Another part of the same volume of hydroxylamine hydrochloride aqueous solution (0.25...
Embodiment 2
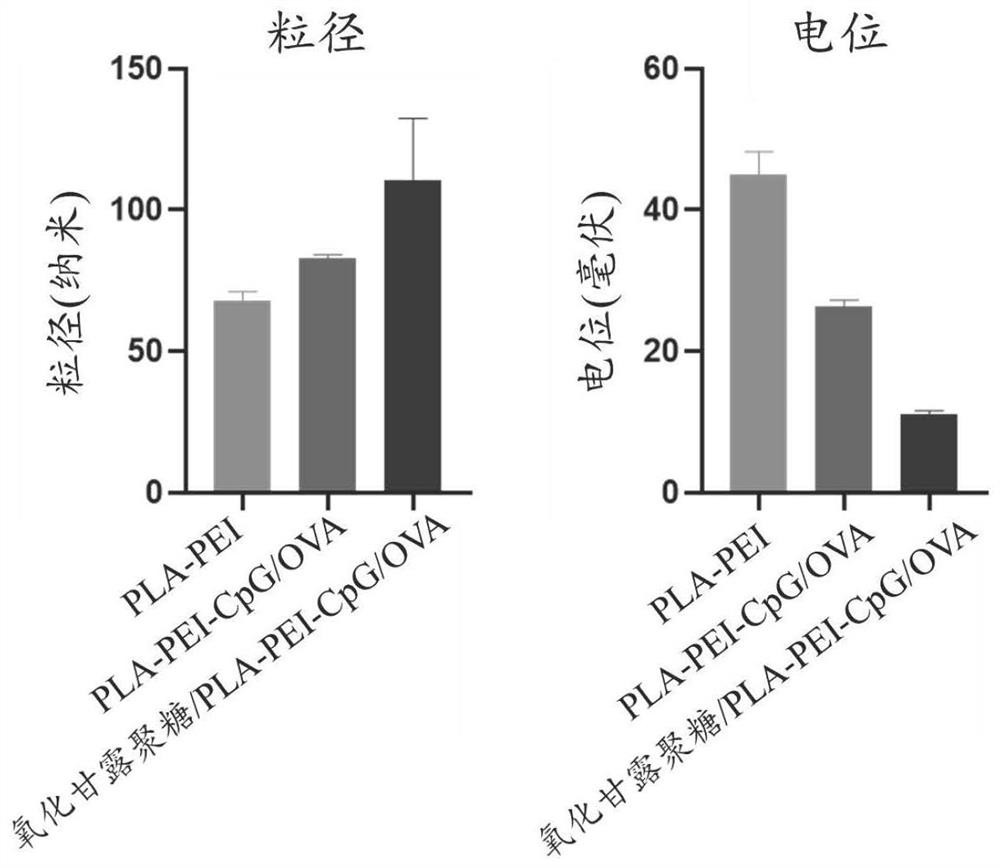
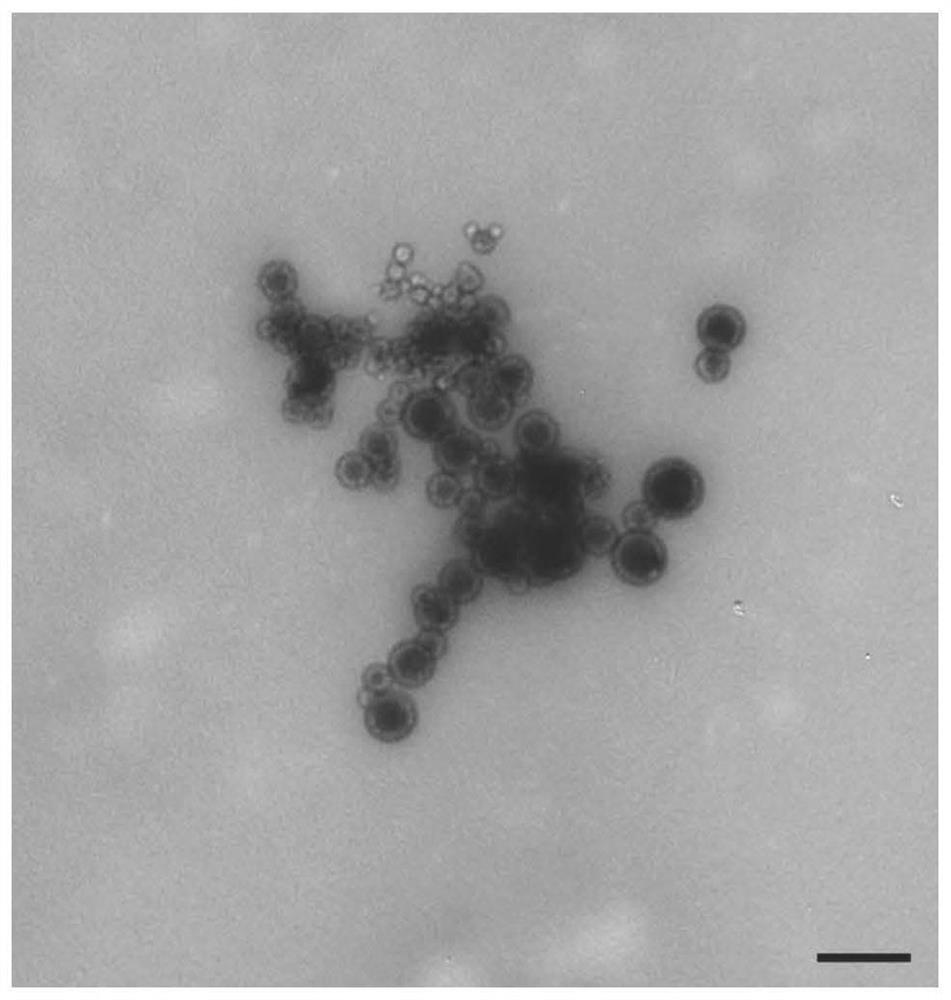
[0052] Preparation of oxidized mannan / PEI-CpG-OVA vaccine
[0053] Dissolve OVA protein in sterile water for injection to obtain an antigen solution with a concentration of 1mg / mL, dissolve PEI in sterile water for injection with a concentration of 1mg / mL, and dissolve CpG in sterile water for injection with a concentration of 1mg / mL, dissolve oxidized mannan in sterile water for injection, the concentration is 1mg / mL, add 1 volume of OVA solution to 4 volumes of PEI solution under vortex, vortex for 30s, and then add 1 volume of The CpG was added to the mixture, vortexed for 30s, and 2 volumes of oxidized mannan solution was added, vortexed for 30s, to obtain the oxidized mannan / PEI-CpG-OVA vaccine. The particle size was measured by a Malvern particle size analyzer and was 211 nm.
Embodiment 3
[0055] Preparation of PEI derivatives (PEI-4BImi)
[0056] 50 mg of PEI (Mw=10kDa) was dissolved in DMSO solution, 25 mg of benzimidazole-7-carboxylic acid, 60 mg of EDC·HCl, and 36 mg of NHS were dissolved in 10 mL of DMSO, stirred at room temperature, activated for 30 min, Added to the DMSO solution of PEI, reacted at room temperature for 72h. The product was purified by dialysis with sterile water for injection, and freeze-dried to obtain the final product PEI-4BImi.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com