Seven-membered heterocyclic compound or salt thereof, and preparation method and application thereof
A technology for seven-membered heterocycles and compounds, which is applied in the field of seven-membered heterocycles or their salts, their preparation and application, can solve problems such as uncertain structures and lack of preparation methods, and achieve the effect of improving safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
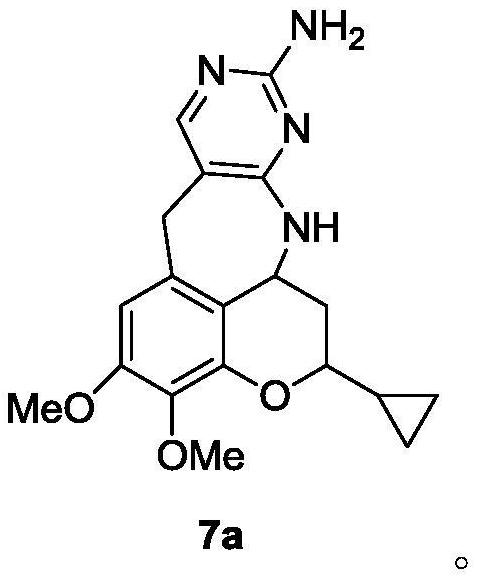
[0037] Example 1 (5-[(2-cyclopropyl-7,8-dimethoxy-2H-1-benzopyran-5-yl)methyl]-2,4-pyrimidine-diamine) preparation
[0038] Step (1) Preparation of (N,N'-(5-(2-acetyl-3,4,5-trimethoxybenzyl)pyrimidine-2,4-diyl)diacetamide)
[0039]
[0040] Add compound 1 (trimethoprim) (10.02g, 34.52mmol), acetic anhydride (17.06g, 167.11mmol) and 100ml chloroform in the 250ml reaction flask, add tin tetrachloride (8.00ml, 68.36mmol) under stirring, Reflux reaction for 1 hour, TLC detected that the raw materials basically disappeared, cooled to room temperature, poured the reaction solution into 50ml of ice water, stirred for 6mins, separated liquids, washed the organic phase with 5ml of water for 3 times, combined the aqueous phase, extracted the aqueous phase with 5ml of chloroform for 3 times, combined the organic phase, adjust the pH to 7-8 with saturated aqueous sodium carbonate solution, separate the liquid, wash the organic phase once with 5ml of water, dry it with anhydrous sodium...
Embodiment 2
[0078] Add compound 6 (1.69g, 4.54mmol) and 20ml tetrahydrofuran to the reaction flask, add trifluoroacetic acid (1.56g, 13.68mmol) under stirring, heat and reflux for 15h, TLC detects that there is basically no raw material remaining, and adjust the pH to 7 or so, tetrahydrofuran was distilled off, the organic phase was adjusted to pH 10 with saturated sodium carbonate solution, stirred for 1 h, filtered with suction, and dried to obtain 1.59 g of compound 7, with a crude yield of 98.87%. HPLC detection (same as Example 1) compound 7 had a purity of 97.01%, wherein HPLC showed that the impurity content was 2.10%, and we obtained 0.021 g by preparative chromatographic column enrichment and separation.
[0079] Its structural identification data are as follows:
[0080] mp.202-204℃; 1 H-NMR (600MHz, DMSO-d 6 ).δ(ppm):7.50(s,1H),6.91(s,1H),6.53(s,1H),5.56(s,2H),5.05-5.04(d,1H),4.41-4.39(d, 1H),3.73(s,3H),3.64(s,3H),3.33-3.30(m,1H),3.17-3.13(d,2H),2.26-2.23(d,1H),1.86-1.82(m, ...
Embodiment 3
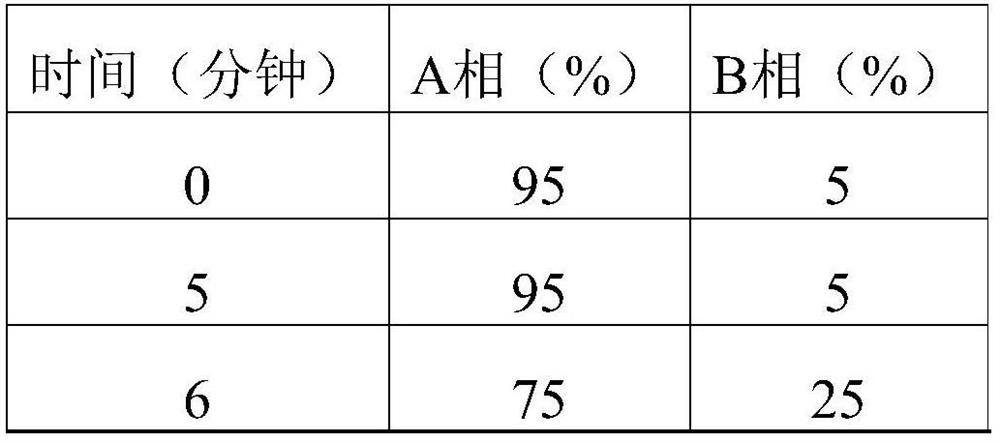
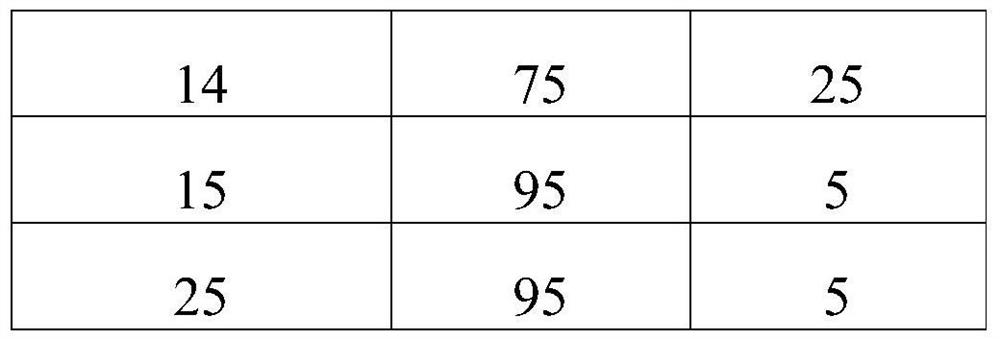
[0086] Add compound 6 (2.00g, 5.38mmol) and 20ml tetrahydrofuran to the reaction flask, add p-toluenesulfonic acid monohydrate (1.54g, 8.10mmol) under stirring, and heat to reflux for 1h. TLC detects that there is basically no raw material remaining, and it drops to At room temperature, adjust the pH to about 7 with saturated sodium carbonate, evaporate THF, adjust the pH to 10 with saturated sodium carbonate solution, stir for 1 h, filter with suction, and dry to obtain 1.81 g of crude compound 7 with a total yield of 95.10%. HPLC detection (HPLC method 2) showed that the purity of compound 7 was 98.25%, and the content of impurity 7a was 1.02%.
[0087] The analysis and identification data (including the peak time of HPLC) are all the same as the above-mentioned Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com