human type-III parainfluenza virus cold-adapted temperature-sensitive strain and application of cold-adapted temperature-sensitive strain
A technology for parainfluenza and cold adaptation, applied in the direction of viruses, antiviral agents, viruses/bacteriophages, etc., to achieve good safety and infection prevention effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
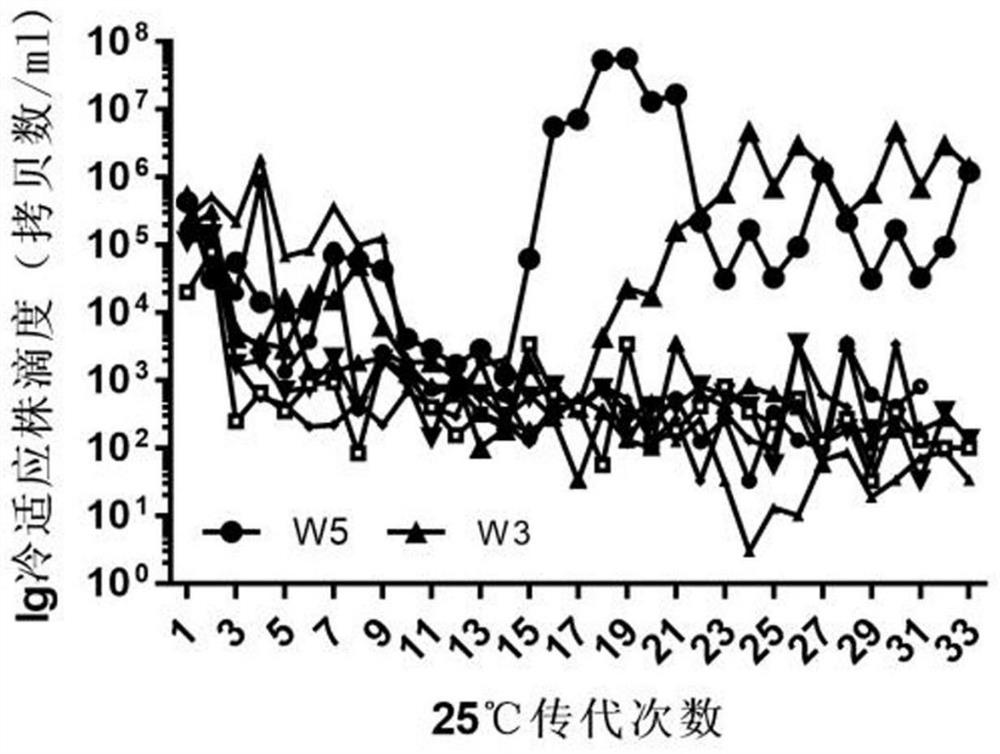
[0030] Example 1 Screening of cold-adapted temperature-sensitive strains of human parainfluenza virus type III
[0031] 1.1 Screening of HPIV-3 positive clinical specimens
[0032] Screening materials: samples of lower respiratory tract secretions from inpatients with lower respiratory tract infection in Maternal and Child Health Hospital of Gansu Province, frozen at -80°C;
[0033] Screening method: Melt the virus screening material at room temperature, then vortex at 12000rpm for 2min; after the concussion, suck the vortex liquid into a 1.5ml centrifuge tube, and centrifuge at 12000rpm for 20min; filter the centrifuged supernatant with a needle filter ( 0.22μm) after sterilizing and filtering, inoculate into 6-well plates grown with WI-38 (human embryonic lung fibroblasts), incubate at 37°C for 2 hours; suck off the supernatant, add 3ml Plasmocin containing 10ug / ml mycoplasma remover (Invivogen, ant-mpt) DMEM medium, placed at 37 ° C, 5% CO 2 Cultivate in the incubator for...
Embodiment 2
[0044] Example 2 Preparation and purification of HPIV3CPW3 virus culture fluid and identification of virus coat protein
[0045] 2.1 Preparation of HPIV3CPW3 virus culture medium
[0046] The HPIV3CPW3 virus strain was inoculated into WI-38 cells at 0.01 MOI, at 25°C, 5% CO 2 After culturing in an incubator for 7 days, the culture supernatant was collected, tested for virus titer by qRT-PCR, and stored at 4°C.
[0047] 2.2 Purification of HPIV3CPW3 virus by ultracentrifugation
[0048] Centrifuge the HPIV3CPW3 virus culture solution prepared in step 2.1 at 8000rpm and 4°C for 30min to precipitate cell debris and collect the supernatant; take 30ml of the supernatant and add it to a 50ml special centrifuge tube, and gently push 15ml50 from the bottom with an 8cm long needle. % sucrose (keep the interface clear during the operation), then put it into an ultracentrifuge (Hitachi cp70mx ultracentrifuge can be selected), and perform ultracentrifugation for 4 hours at 4°C and 35000...
Embodiment 3
[0053] Example 3 Determination of the Whole Gene Sequence of HPIV3CPW3 and Analysis of Genetic Characteristics
[0054] The whole gene sequence of HPIV3CPW3 was determined by next-generation sequencing, and the specific process was completed by Shanghai Bojie Medical Technology Co., Ltd. Sequencing results show that the full gene sequence of HPIV3CPW3 contains 15462 nucleotides. According to nucleotide sequence analysis, the homology between HPIV3CPW3 and HPIV-3 isolate MH678682.1 on GenBank is 99.87%. The sequence conforms to the "6-base principle" of the Paramyxoviridae genome, according to 5'-NP (111-1658)-PP / C(1784-3592)-M(3753-4814)-F(5072-6691)-HN( The sequence of 6806-8524)-L (8571-15347)-3' encodes 6 proteins, and the gene structure is the same as that of the HPIV-3 isolate with known sequence. Description HPIV3CPW3 strain is a type III parainfluenza virus.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com