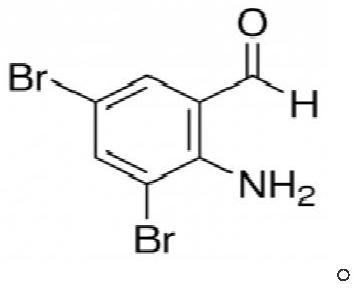
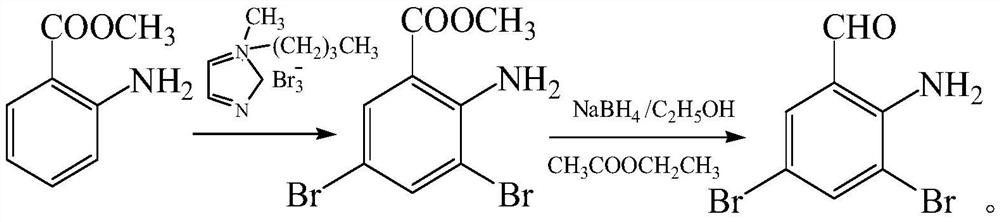
Production process of 2-amino-3,5-dibromobenzaldehyde
A technology of anthranilaldehyde and its production process, which is applied in the field of medicine and chemical industry, can solve the problems of o-nitrodibromobenzyl not being effectively and rationally utilized, waste of raw materials, and increased cost of waste treatment, so as to realize high-value transformation and promote The effect of reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
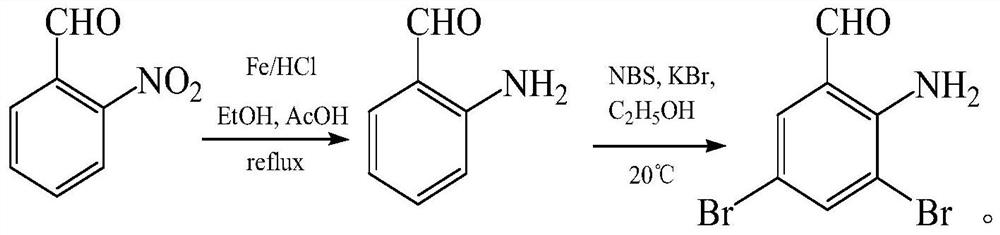
[0038] In this embodiment 1, o-nitrodibromobenzyl is prepared into o-aminodibromobenzyl extraction reaction solution, comprising the following steps:
[0039] Add 100ml of water, 0.4005mol active iron powder and 0.0097mol hydrochloric acid solution into the reaction flask, stir at room temperature for 30 minutes, add 50g toluene and 0.1695mol o-nitrodibromobenzyl, heat and reflux to HPLC to detect the completion of the o-nitrodibromobenzyl reaction Afterwards (use time 3h), add 5% sodium carbonate aqueous solution to adjust pH=7-9 in the reaction bottle, filter at the temperature of 60-75 ℃ to obtain the filtrate, put the filtrate in the separatory funnel and let it stand for liquid separation. 90.5 g of the o-aminodibromobenzyltoluene reaction solution was obtained, and the purity detected by HPLC was 95%.
Embodiment 2
[0041] In this embodiment 2, o-nitrodibromobenzyl is prepared into o-aminodibromobenzyl extraction reaction solution, comprising the following steps:
[0042] Add 100ml of water, 0.4205mol active iron powder and 0.0097mol hydrochloric acid solution into the reaction flask, stir at room temperature for 30 minutes, add 50g toluene and 0.1695mol o-nitrodibromobenzyl, heat and reflux to HPLC to detect the completion of the o-nitrodibromobenzyl reaction Afterwards (use time 2h), add 5% sodium carbonate aqueous solution to adjust pH=7-9 in the reaction flask, filter at the temperature of 60-75 ℃ to obtain the filtrate, put the filtrate in the separatory funnel and let it stand for liquid separation. 91.5 g of the o-aminodibromobenzyltoluene reaction solution was obtained, and the HPLC detection purity was 96.2%.
Embodiment 3
[0044] In this embodiment 3, o-nitrodibromobenzyl is prepared into o-aminodibromobenzyl extraction reaction liquid, comprising the following steps:
[0045] Add 100ml of water, 0.4205mol of active iron powder and 0.0097mol of hydrochloric acid solution into the reaction flask, stir at 70-80°C for 30 minutes, then cool down to room temperature, add 50g of toluene and 0.1695mol of o-nitrobenzyl bromide, heat and reflux to detect o-nitrobenzyl bromide by HPLC After the reaction of dibromobenzyl bromide is completed (taking 2h), add 5% aqueous sodium carbonate solution to the reaction flask to adjust the pH=7-9, filter at a temperature of 60-75°C to obtain the filtrate, and place the filtrate in a separatory funnel After standing still for liquid separation, 92.1 g of the o-aminodibromobenzyltoluene reaction solution was separated, and the purity detected by HPLC was 96.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com