Application of aspartic protease gene in improvement of beauveria bassiana variety
A technology of Beauveria bassiana and aspartic acid, which is applied in the direction of plant genetic improvement, application, genetic engineering, etc., can solve problems such as unreported high temperature tolerance, achieve improved yield and virulence, and improve high temperature tolerance effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, BbASP gene cloning
[0036] Design upstream and downstream primers according to the aspartic protease sequence in NCBI:
[0037] ASP-F: 5'-ATGTCGCTCAGAAACATCGTC-3' (SEQ ID NO.1);
[0038] ASP-R: 5'-TTACTTGAGATTAGCGAAGCC-3' (SEQ ID NO.2);
[0039] Using the genomic DNA of wild-type Beauveria Bassiana Bb0062 as a template, use Max DNA Polymerase was used for PCR amplification, and the product was gel-recovered after gel electrophoresis to verify that the size was correct, and then the fragment was connected to the cloning vector pEASY-Blunt, and the BbASP gene was obtained after enzyme digestion and sequencing were verified to be correct.
[0040] The full length of BbASP genomic DNA is 1208bp (SEQ ID NO.3), containing two introns, the full length of ORF sequence is 1068bp (SEQ ID NO.4), encoding 355 amino acids (SEQ ID NO.5), and the predicted protein molecular weight is 37.5kDa.
Embodiment 2
[0041] Example 2, Expression analysis of BbASP gene under high temperature stress
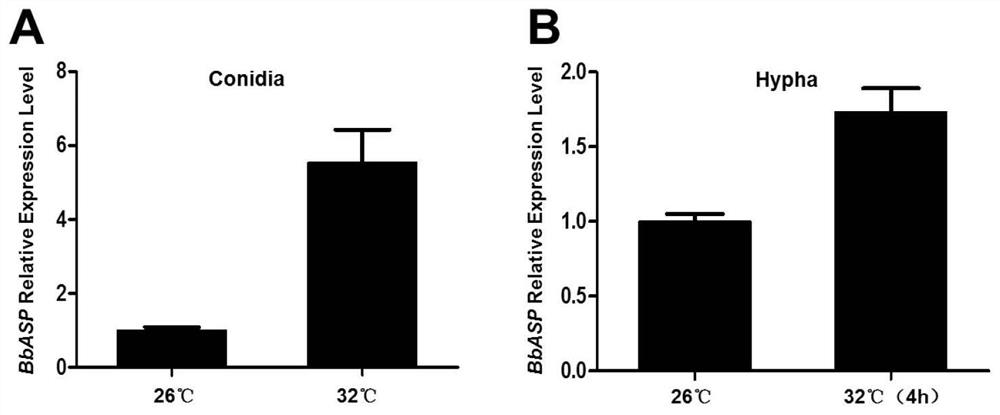
[0042] Two culture methods A and B were used to detect the difference in the expression of BbASP gene in the conidia and hyphae of Beauveria bassiana under normal temperature (26°C) and high temperature (32°C) stress.
[0043] (A) Take 50μL concentration of 1×10 7 Conidia / mL wild-type spore suspension of Beauveria bassiana was placed on CZM solid medium, and cultured at 26°C and 32°C for 14 days respectively, then the conidia RNA was extracted and reverse-transcribed into cDNA, and the γ-Actin gene was used as the The expression level of BbASP gene was detected by internal standard, and no stress was used as the control;
[0044] (B) Take 50μL concentration of 1×10 7 Conidia / mL wild-type spore suspension of Beauveria bassiana was added to 1 / 4 SDY liquid medium, cultured on a shaker at 26°C and 200rpm for 3 days, then stressed at 32°C for 4 hours, and mycelial RNA was extracted and reverse tra...
Embodiment 3
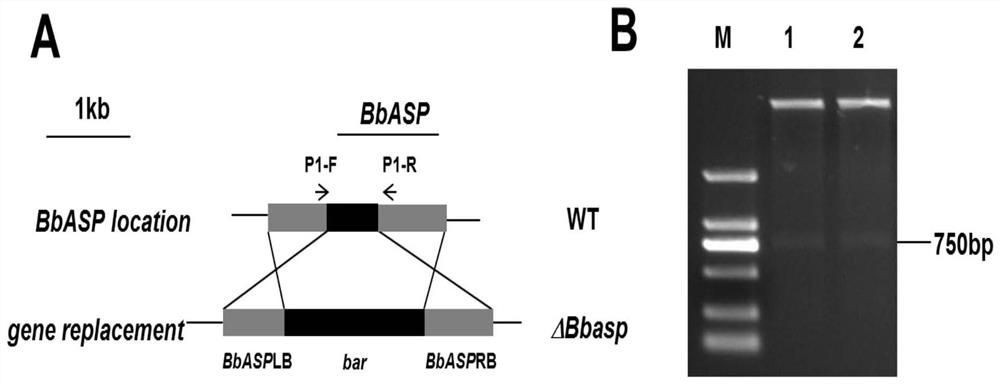
[0051] Embodiment 3, construction of BbASP gene homologous knockout vector
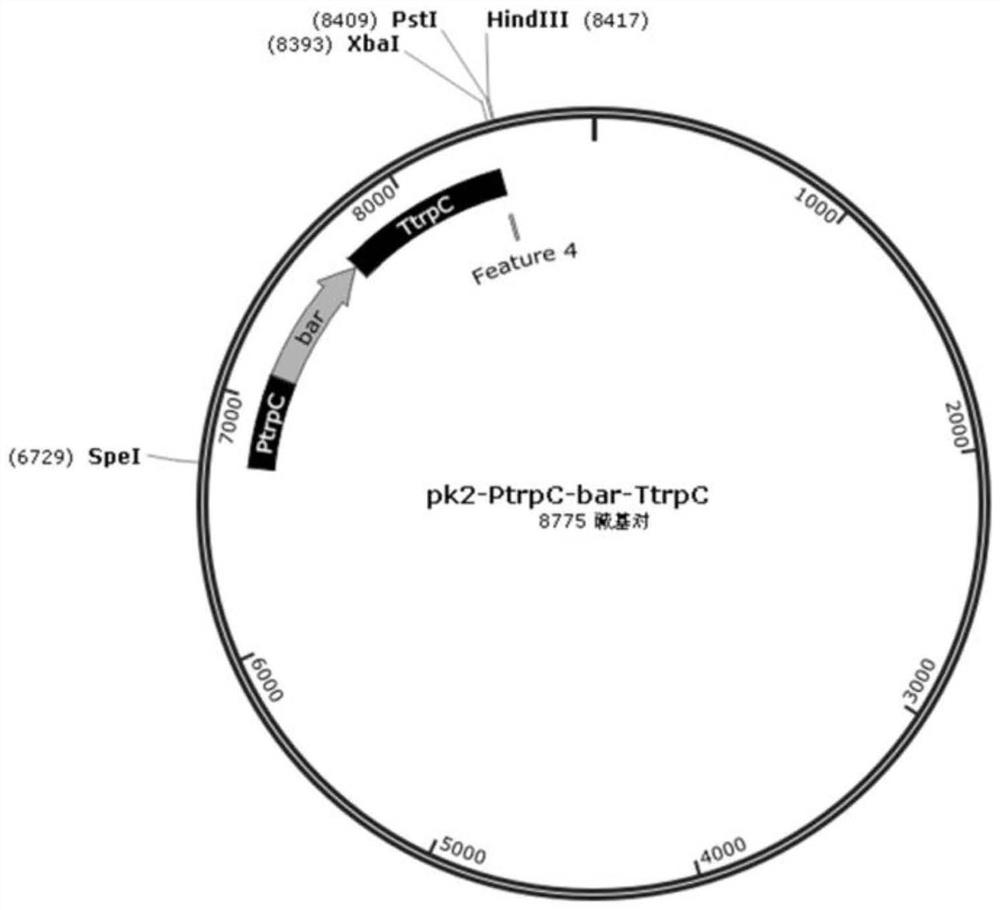
[0052] Homologous knockout backbone vector selection pK 2 -PtrpC-bar-TtrpC (referred to as pK 2 -bar), which was developed by our lab in the backbone vector pK 2 Based on the transformation obtained, pK 2 -PtrpC-bar-TtrpC vector schematic diagram as figure 2 As shown, bar is a glufosinate-resistant gene, and the nucleotide sequence of the bar gene is shown in SEQ ID NO.10. A sequence was selected at the 5' end and 3' end of the ORF region of the BbASP gene as a homology arm, and primers were designed , the underline represents the enzyme cleavage site:
[0053] LB-F: 5'-CG GAATTC GCATGCTCACTTTGCAACTTC-3' (EcoRI, SEQ ID NO.11);
[0054] LB-R: 5'-CG GAATTC CGATGACTGACGCTGAATTG-3' (EcoRI, SEQ ID NO. 12);
[0055] RB-F: 5'-GC TCTAGA GGTCAAGTTTATCGCGACGTC-3' (XbaI, SEQ ID NO. 13);
[0056] RB-R: 5'-CCC AAGCTT CTTTGCAGCAATCCATGAGTC-3' (Hind III, SEQ ID NO. 14);
[0057] The fragments of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com