Benzothiadiazole-based acceptor material, and preparation method and application thereof
A technology based on benzothiadiazole and benzothiadiazole thickening, applied in the field of designing organic photoelectric materials, can solve problems such as limited absorption range, unsatisfactory short-circuit current, and enhanced molecular spectral absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of ultra-narrow bandgap acceptor material 1V-2F
[0028]
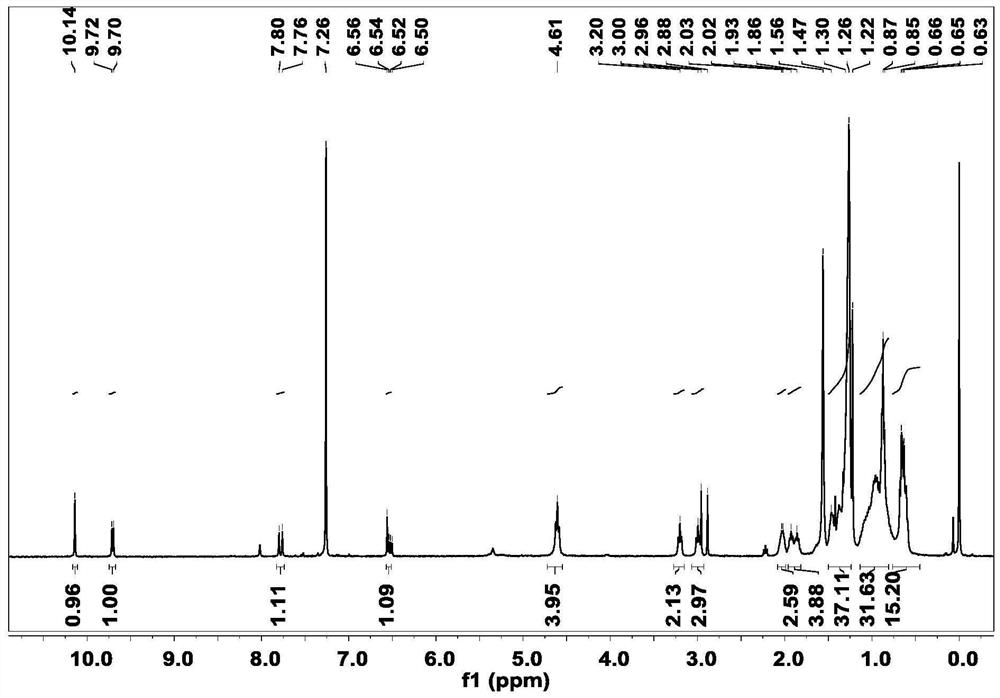
[0029] Synthesis of Preparation Intermediate 1: Dissolve the hydroformylated seven-membered benzothiadiazole fused-ring nucleus (560 mg, 0.5 mmol) (purchased from Suzhou Nakai Company) in 30 mL of anhydrous tetrahydrofuran, and add 1 equivalent of the hydroformylated seven-membered benzene Tributyl(1,3-dioxan-2-ylmethyl)phosphonium bromide (180mg, 0.5mmol) and sodium hydride (24mg, 0.5mmol) with thiadiazole fused ring nucleus, stirring at room temperature under nitrogen protection After 8 hours, 10 mL of dilute hydrochloric acid was added, extracted with dichloromethane, washed with deionized water, dried over anhydrous sodium sulfate and spin-dried, and purified by column chromatography to obtain intermediate 1. 1 H NMR (400MHz, CDCl 3 )δ10.14(s,1H),9.71(d,J=7.6Hz,1H),7.78(d,J=15.3Hz,1H),6.77-6.28(m,1H),4.61(t,J=7.7 Hz, 4H), 3.20(t, J=7.5Hz, 2H), 2.99(t, J=7.5Hz, 2H), 2.04(s, 2H), 1.99-1.83(m, 4H...
Embodiment 2
[0037] Synthesis of ultra-narrow bandgap acceptor material 3V-2F
[0038]
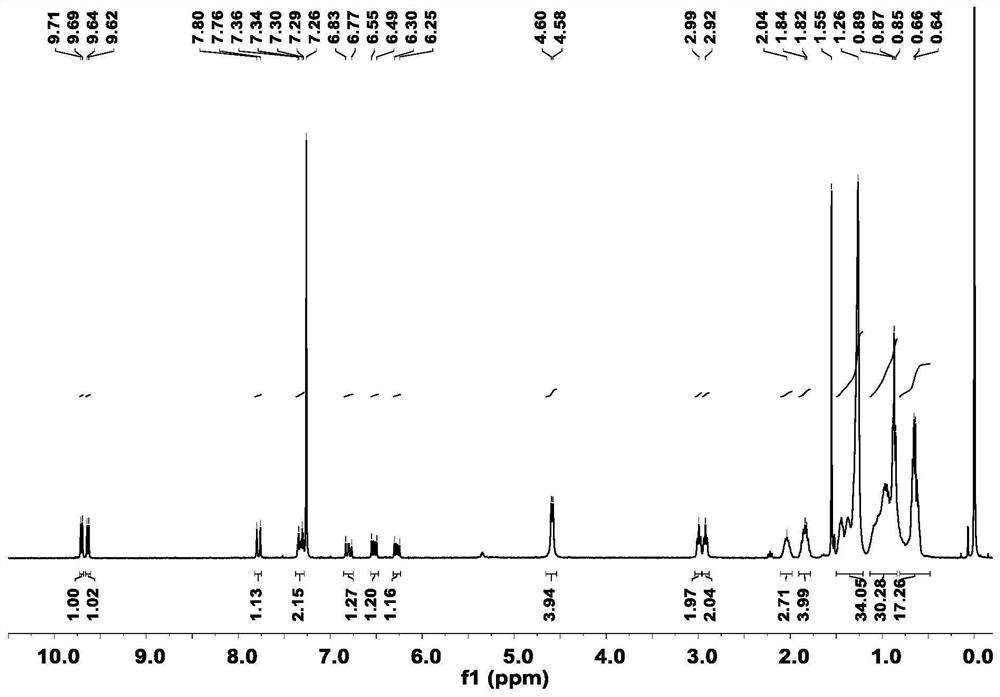
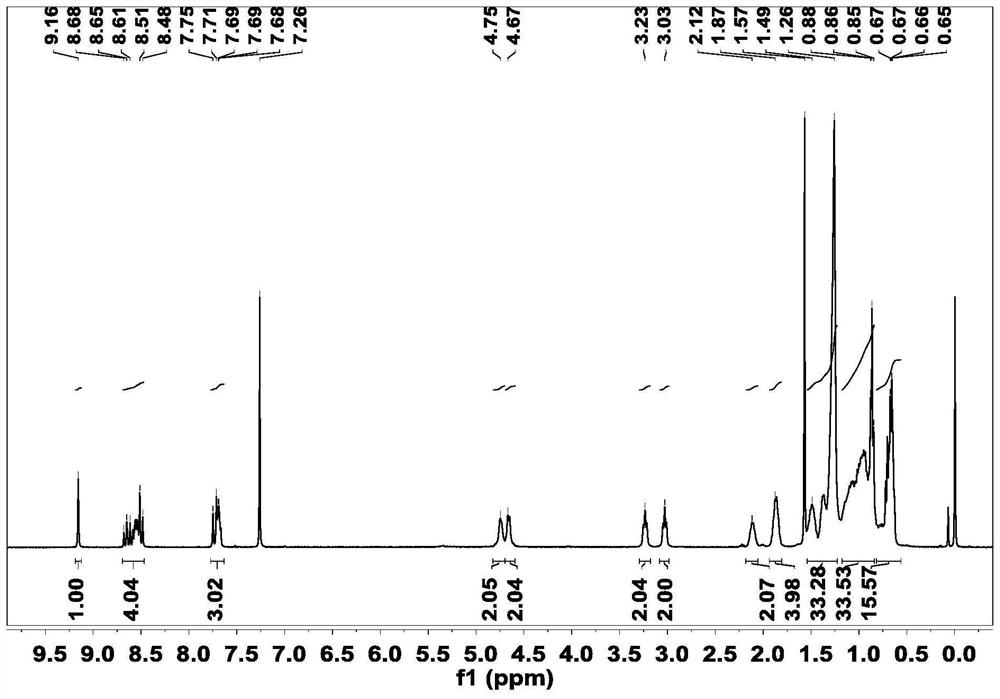
[0039]Synthesis of acceptor material 3V-2F: Intermediate 2 (69mg, 0.06mmol) and 2(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-1-ethylene ) malononitrile (52 mg, 0.22 mmol) was dissolved in 20 mL of chloroform solution, 1 ml of anhydrous pyridine was added, and reacted at 65° C. for 10 h under nitrogen protection. After the reaction, the solution was poured into methanol, and the precipitated solid was dissolved, extracted, dried, and purified by column chromatography to obtain the final product 3V-2F. 1 H NMR (400MHz, Chloroform-d) δ8.72-8.58(m,1H),8.58-8.46(m,3H),8.53-8.39(m,1H),8.33-8.20(m,1H),7.68(m ,3H),7.53-7.39(m,1H),7.42-7.30(m,1H),7.15-6.95(m,1H),4.64(s,4H),3.10-2.85(m,6H),2.10(s ,1H),1.96-1.78(m,4H),1.52-1.24(m,32H),1.19-0.82(m,30H),0.69(dd,J=16.5,6.9Hz,18H).MS(MALDI-TOF )m / z calcd.for(C 96 h 108 f 4 N 8 o 2 S 5 ):1641.7168. Found: 1641.7151.
Embodiment 3
[0041] Measuring the Optical Band Gap of Acceptor Material 1V-2F Using Absorption Spectroscopy
[0042] The acceptor material 1V-2F prepared in Example 1 is measured under chloroform and thin film UV-visible absorption spectrum as Figure 4 shown. The optical bandgap of the acceptor material can be determined by the empirical formula (E g opt =1240 / λ 吸收边缘 ) is calculated to be 1.26eV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com