A kind of organic photovoltaic material based on perylene imide and its preparation method and application
An organic photovoltaic material, nitroperylene imide technology, applied in the fields of organic chemistry, photovoltaic power generation, semiconductor/solid-state device manufacturing, etc., can solve the problem that the performance of non-fullerene electron acceptor materials is difficult to effectively control and the light absorption effect is not good. Ideal, complex material preparation method and other problems, to achieve the effect of low synthesis cost, fewer synthesis steps, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the preparation of PDINCB
[0041]
[0042] PDI-NO 2 : PDI (10g, 14.3mmol) was dissolved in 500mL of dichloromethane, fuming nitric acid (10mL) was added dropwise in the above PDI solution, stirred at room temperature, the reaction solution was washed with sodium hydroxide, extracted three times with dichloromethane , with MgSO 4 Drying followed by purification by column chromatography afforded PDI-NO 2 (8 g), 75% yield. Such as figure 1 as shown, 1 H NMR (400MHz, CDCl 3,δ,ppm):9.78(d,J=8.3Hz,1H),8.92(s,1H),8.64(t,J=17.8Hz,5H),5.17(qd,J=9.7,4.7Hz,2H) ,2.30-2.16(m,4H),1.91-1.77(m,4H),1.36-1.22(m,24H),0.83(t,J=6.8Hz,12H). 13 C NMR (101MHz, CDCl 3 ,δ,ppm):164.32(s),165.05-161.78(m),161.78-160.77(m),147.68(s),135.46(s),133.29(s),132.94(s),132.24(d,J =134.2Hz), 131.13(s), 129.56-129.04(m), 127.90(s), 127.74-126.33(m), 126.14(s), 124.47(s), 124.01(s), 55.25(s), 54.96 (s), 32.22(d, J=9.0Hz), 31.69(d, J=2.6Hz), 26.58(d, J=1.9Hz), 23.09-22.15(m), 14.02...
Embodiment 2
[0047] Embodiment 2: Preparation of 2PDINCB
[0048]
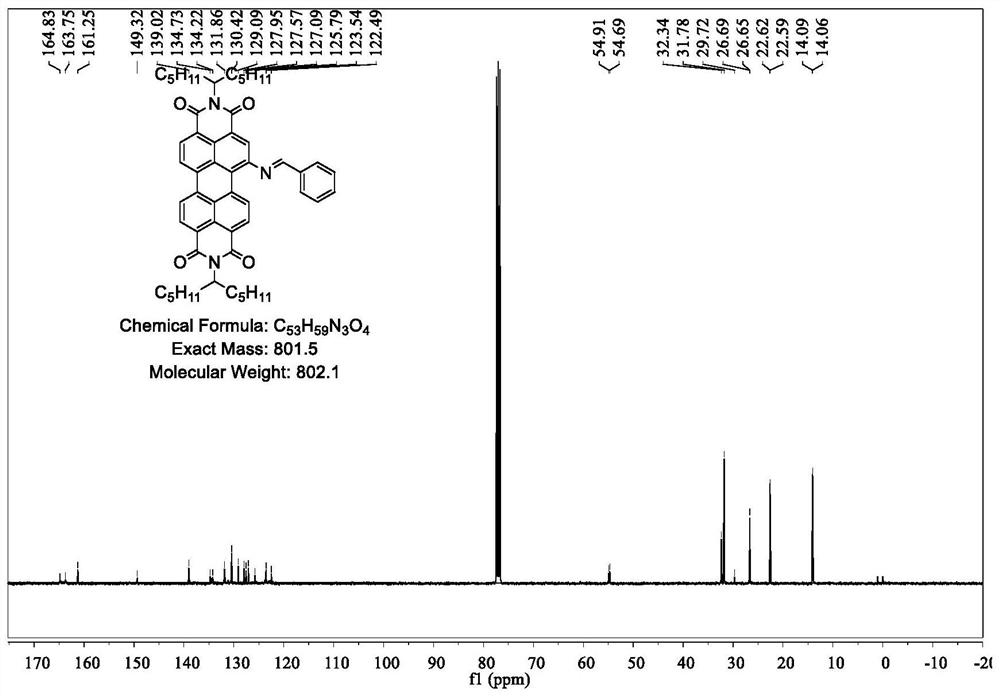
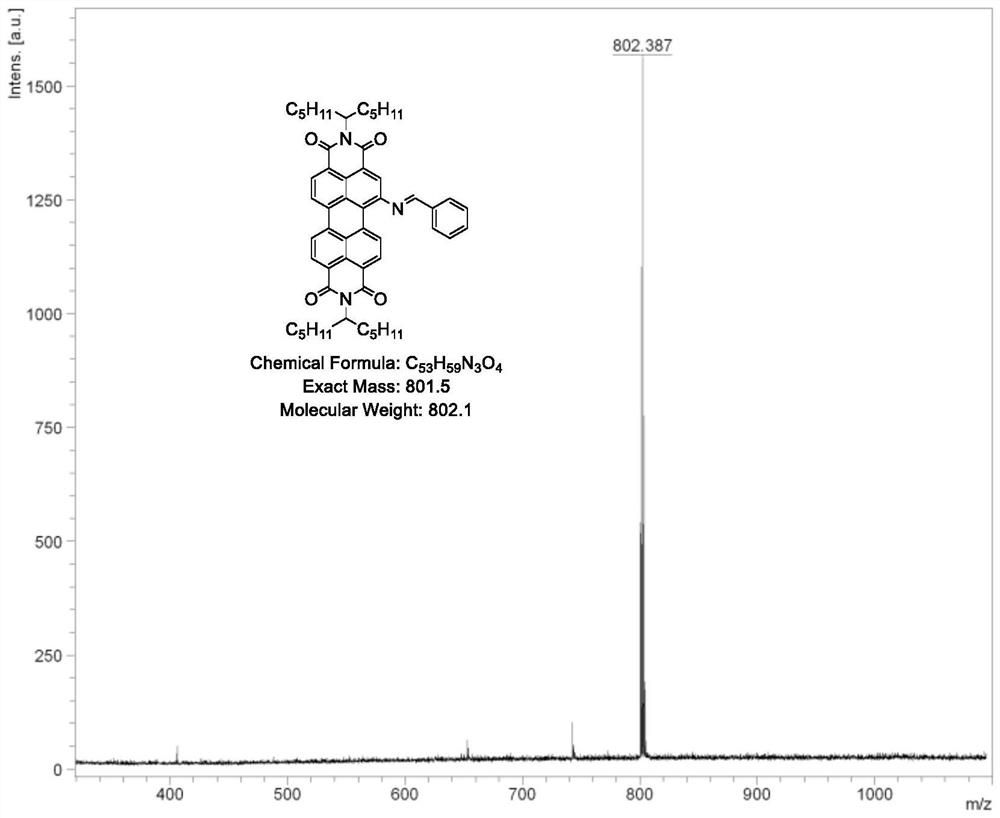
[0049] 2PDINCB: PDI-NH 2 (1g, 1.4mmol) and terephthalaldehyde (54mg, 0.4mmol) were dissolved in absolute ethanol, added 1mL acetic acid, stirred overnight at 80°C, extracted with DCM, MgSO 4 Drying and purification by column chromatography afforded 2PDINCB (964 mg) in 91% yield. Such as Figure 4 as shown, 1 H NMR (400MHz, CDCl 3 ,δ,ppm)9.11(d,J=8.3Hz,2H),8.99(s,2H),8.78-8.66(m,10H),8.38(s,6H),5.27-5.16(m,4H),2.28 (dd,J=15.5,6.1Hz,8H),1.92-1.81(m,8H),1.29(dd,J=17.3,6.0Hz,48H),0.84(dd,J=13.4,6.7Hz,24H). Such as Figure 5 as shown, 13 C NMR (101MHz, CDCl 3 ,δ,ppm)164.81(d,J=11.5Hz),163.72(d,J=5.8Hz),161.25(s),149.32(s),139.02(s),134.94-134.17(m),131.86(s ),131.13(s),130.42(s),129.09(s),127.95(s),127.57(s),127.09(s),125.79(s),123.54(s),122.49(s),55.21-54.99 (m), 54.80(d, J=22.0Hz), 32.34(s), 31.78(s), 29.72(s), 26.67(d, J=4.0Hz), 22.60(d, J=2.8Hz), 14.08 (d, J=3.0Hz). Such as Figure 6 Shown, Calcd for MS: 1524...
Embodiment 3
[0050] Embodiment 3: Preparation of PDINCF
[0051]
[0052] PDINCF: PDI-NH 2 (1g, 1.4mmol) and fluorenaldehyde (256mg, 4mmol) were dissolved in absolute ethanol, added 1mL acetic acid, stirred overnight at 80°C, extracted with DCM, MgSO 4 After drying and purification by column chromatography, PDINCF (0.97 g) was obtained in 93% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com