Alpha-position vinyl bridged BODIPY conjugated polymer and preparation method thereof
A conjugated polymer, ethylene technology, applied in the fields of organic synthesis, dyes and fine chemicals, can solve the problems of limited application, difficult to achieve effective absorption, etc., achieve narrow optical band gap, is conducive to mass production, full application potential effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072]
[0073] Under an argon atmosphere, 0.100 mmol of 1a monomer, 0.102 mmol of 1b monomer, 0.003 mmol of tris(dibenzylideneacetone)dipalladium [Pd 2 (dba) 3 ] with 0.012 mmol tris(o-methylphenyl)phosphine [P(o-Tol) 3 ] ligand, take 6 mL of toluene (Toluene) into the bottle, heat up to 120 ° C, stir the reaction under argon protection for 24 hours, then cool to room temperature, pour the reaction solution into methanol, filter the precipitated solid, vacuum dry, The polymer was washed with acetone and n-hexane successively to remove small molecules and catalysts with a Soxhlet extractor, and finally the polymer was extracted with chloroform. Calculated yield: 92%.
[0074] The BODIPY class monomer 1a in Example 1 is detected by hydrogen NMR and carbon NMR, and the results are as follows: Figure 9 and Figure 10 As shown, the ethylene double tin salt monomer 1b in Example 1 was detected by hydrogen nuclear magnetic spectrum, and the results were as follows Figure 1...
Embodiment 2
[0079]
[0080] Under an argon atmosphere, 0.150 mmol of 2a monomer, 0.600 mmol of piperidine and 0.100 mmol of acetic acid were added to a 20 mL pressure bottle, 5 mL of toluene (Toluene) was added to the pressure bottle, and the temperature was raised to 120 ° C. The reaction was stirred for 48 hours, then cooled to room temperature, the reaction solution was poured into methanol, the precipitated solid was filtered, dried in vacuum, the polymer was washed with a Soxhlet extractor with acetone and n-hexane in turn to remove small molecules and catalysts, and finally Chloroform extracted the polymer. Calculated yield: 79%.
[0081] Carry out hydrogen nuclear magnetic spectrum to the BODIPY monomer 2a in embodiment 2, the results are as follows respectively Figure 12 shown.
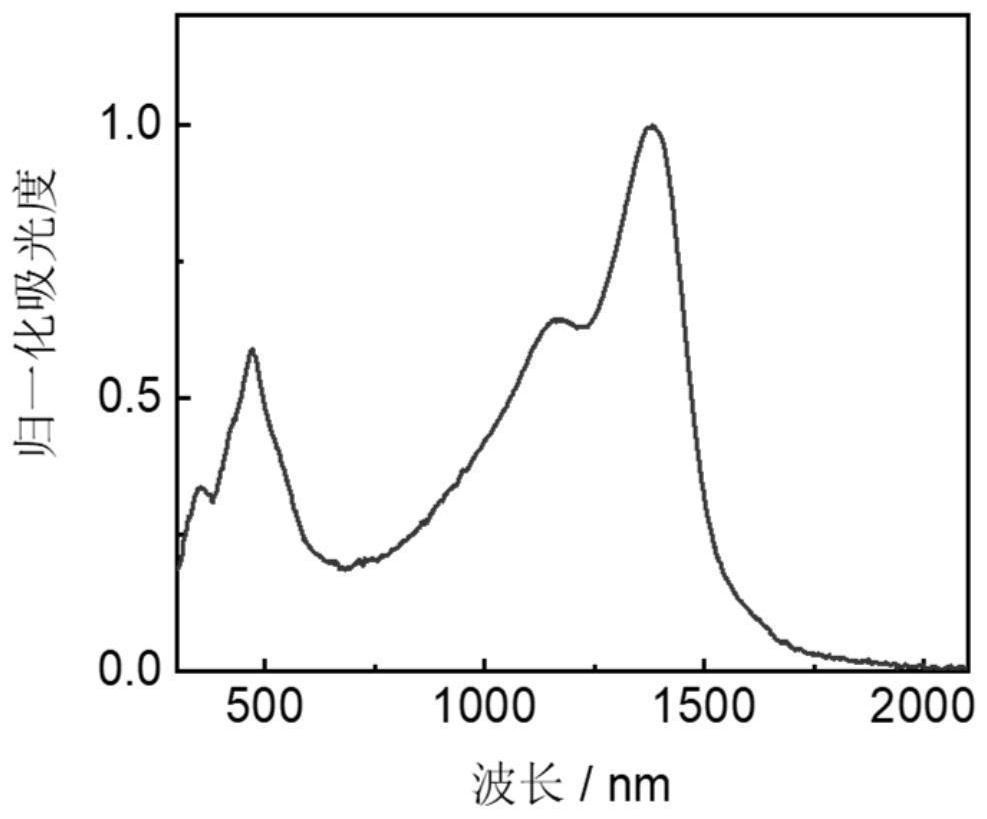
[0082] Absorption spectrum analysis is carried out to the macromolecule prepared in Example 2, and the test result is as follows Figure 5 shown.
[0083] The prepared polymer was analyzed by gel ...
Embodiment 3
[0087]
[0088] Under an argon atmosphere, 0.100 mmol of 3a monomer, 0.100 mmol of 3b monomer, 0.003 mmol of tris(dibenzylideneacetone)dipalladium [Pd 2 (dba) 3 ] with 0.012 mmol tris(o-methylphenyl)phosphine [P(o-Tol) 3 ] ligand, add 6 mL of toluene (Toluene) into the bottle, heat up to 120°C, stir the reaction under argon for 24 hours, then cool to room temperature, pour the reaction solution into methanol, filter the precipitated solid, and vacuum dry, The polymer was washed with acetone and n-hexane successively to remove small molecules and catalysts with a Soxhlet extractor, and finally the polymer was extracted with chloroform. Calculated yield: 89%.
[0089] The BODIPY class monomer 3a in Example 3 is detected by hydrogen NMR and carbon NMR, and the results are as follows respectively. Figure 13 and Figure 14 shown.
[0090] The absorption spectrum of the polymer prepared in Example 3 is as follows Figure 7 shown.
[0091] The prepared polymer was analyzed b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com