Long-chain branched degradable polyester and preparation method thereof
A technology for degrading polyester and long-chain branching, which is applied in the production of bulk chemicals, plastic recycling, and recycling technology. The effect of degradation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] A preparation method of the degradable polyester, comprising the following steps:
[0050](1) Esterification reaction: Put all the monomers, catalysts and stabilizers into the reaction container, and after removing the air, carry out the esterification reaction at 180-260°C and 0.2-0.4MPa; when the amount of esterification distillate reaches the theoretical When the value is 75-85%, the esterification reaction is ended;
[0051] The catalyst is stannous octoate, stannous isooctanoate, stannous oxalate, stannous chloride, stannous oxide, antimony ethylene glycol, antimony trioxide, antimony acetate, tetrabutyl titanate, n-tetrapropyl titanate , one or more of titanium oxalate, titanium acetate and titanium tetrachloride; the amount of catalyst used is 50-400 ppm of the molar weight of all organic acids and their derivatives;
[0052] The stabilizer is one or more of phosphoric acid, alkyl phosphate, triphenyl phosphate, alkyl diaryl phosphate and mixed alkyl aryl phosph...
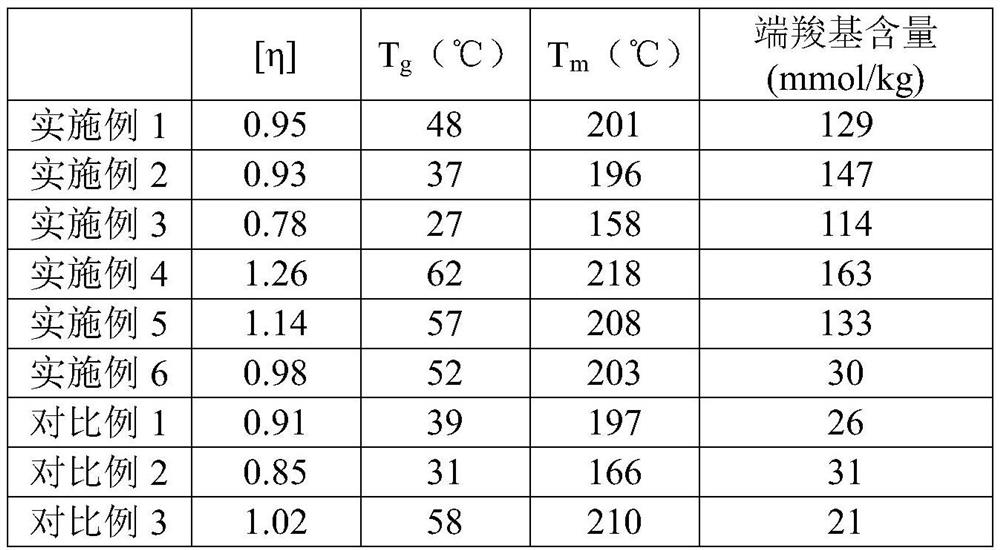
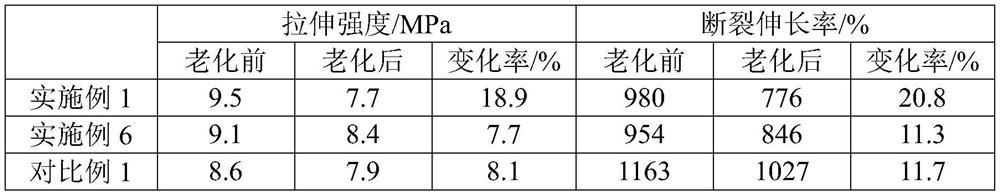
Embodiment 1
[0056] A preparation method for long-chain branched degradable polyester, comprising the following steps:
[0057] (1) Esterification reaction: put 415.33g terephthalic acid, 365.35g adipic acid, 8.41g trimellitic acid, 545.05g butanediol, 0.17g tetrabutyl titanate and 0.82g triphenyl phosphate into the reaction In the kettle, use nitrogen to remove the air in the reaction kettle, and carry out the esterification reaction at 250°C and 3MPa; when the amount of the esterification distillate reaches about 80% of the theoretical value, the esterification reaction is terminated;
[0058] (2) Polycondensation reaction: Vacuumize the glycol to remove the diol, and make the vacuum degree reach within 60Pa after 60 minutes. At the same time, adjust the temperature to 280°C and carry out the polycondensation reaction until a certain intrinsic viscosity is reached to obtain a long-chain branched degradable polymer. ester.
Embodiment 2
[0060] A preparation method for long-chain branched degradable polyester, comprising the following steps:
[0061] (1) Esterification reaction: put 498.39g terephthalic acid, 292.28g adipic acid, 8.41g trimellitic acid, 545.05g butanediol, 0.17g tetrabutyl titanate and 0.82g triphenyl phosphate into the reaction In the kettle, use nitrogen to remove the air in the reaction kettle, and carry out the esterification reaction at 250°C and 3MPa; when the amount of the esterification distillate reaches about 80% of the theoretical value, the esterification reaction is terminated;
[0062] (2) Polycondensation reaction: Vacuumize the glycol to remove the diol, and make the vacuum degree reach within 60Pa after 60 minutes. At the same time, adjust the temperature to 290°C and carry out the polycondensation reaction until a certain intrinsic viscosity is reached to obtain a long-chain branched degradable polymer. ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com