A kind of synthetic method of c-glycoside derivative
A synthesis method and a technology of derivatives, which are applied in the field of synthesis of C-glycoside derivatives, can solve the problems of unfavorable large-scale industrial production, boron trifluoride ether is flammable and explosive, and complicated processes, so as to avoid the growth of impurities, The quenching time is short and the effect of ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] This example is used to illustrate the method of synthesizing and purifying the compound of formula (I) starting from the compound of formula (II), and further synthesizing the L-proline co-crystal complex of the compound of formula (I).
[0050] 1. Under the protection of nitrogen, add dichloromethane (364.1 kg) into a 500 L cryogenic reaction kettle, start stirring, and add triethylsilane (20.3 kg) and compound of formula (II) (28.1 kg). Cool down to -40°C. 20% boron trifluoride acetonitrile complex (69.1 kg) was added dropwise under temperature control for about 2 hours. After dropping, keep warm for 3 hours to synthesize the compound of formula (I).
[0051] 2. Add 5% sodium bicarbonate aqueous solution (313.73kg) into the 1000 L cryogenic reaction kettle. The feed liquid in the 500 L cryogenic reactor kept at -40°C was directly dropped into the 1000 L cryogenic reactor to quench the reaction (dropping for about 1 hour), and the temperature in the 1000 L cryogenic...
Embodiment 2
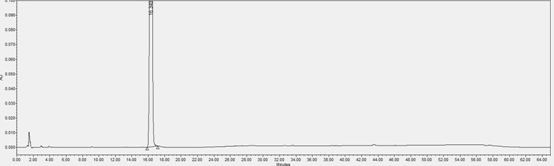
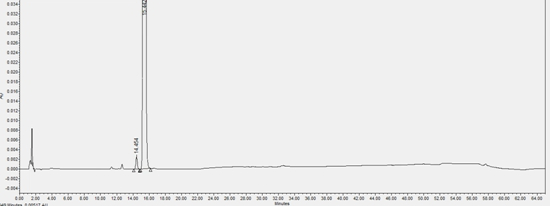
[0059] In this example, the content of impurity (X) in the L-proline eutectic complex of the compound of formula (I) prepared in Example 1 was detected by HPLC.
[0060] The detection method is as follows:
[0061] Reagent solution: diammonium hydrogen phosphate, phosphoric acid, methanol, acetonitrile, ultrapure water.
[0062] Instruments and equipment: electronic balance, high performance liquid chromatography, pH meter, centrifuge.
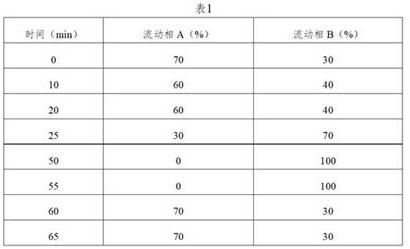
[0063] Chromatographic conditions: determined according to high-performance liquid chromatography (Chinese Pharmacopoeia 2020 Edition Sibu General Rules 0512), using octadecylsilane-bonded silica gel as a filler column (Agilent Eclipse XDB-C18, 150mm×4.6mm, 5μm); Mobile phase A is 0.01mol / L diammonium hydrogen phosphate solution (take 1.32g of diammonium hydrogen phosphate, add 1000mL of water to ultrasonically dissolve, adjust the pH value to 5.00±0.05 with phosphoric acid)-acetonitrile (90:10), mobile phase B is Water-acetonitrile (10:90);...
Embodiment 3
[0068] In this example, according to the synthesis method described in Example 1, three batches of samples were respectively prepared with different amounts of starting materials, and the samples were subjected to high-efficiency liquid phase detection, and the results are as follows:
[0069]
[0070] Experimental results prove that the synthetic method provided by the present invention has good repeatability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com