Albendazole-cholic acid derivative as well as preparation method and application thereof
A technology for albendazole and derivatives, applied in the field of albendazole-cholic acid derivatives and their preparation, can solve the problems of unstable bioavailability, low water solubility, influence on treatment and the like, and achieves improved transport efficiency, The effect of increasing solubility and reducing crystallization tendency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
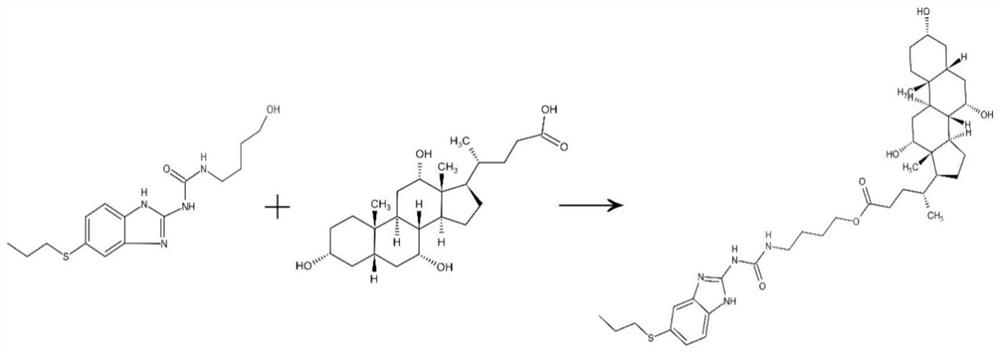
[0052] An albendazole-cholic acid derivative, made from raw materials comprising the following components and contents: 6g of albendazole; 5mL of cross-linking agent; 54mL of N,N-dimethylformamide; 20g of cholic acid; Dicyclohexylcarbodiimide 8g; 4-dimethylaminopyridine 0.5g.
[0053] Preparation of albendazole-cholic acid derivatives
[0054] S1, preparation of intermediates
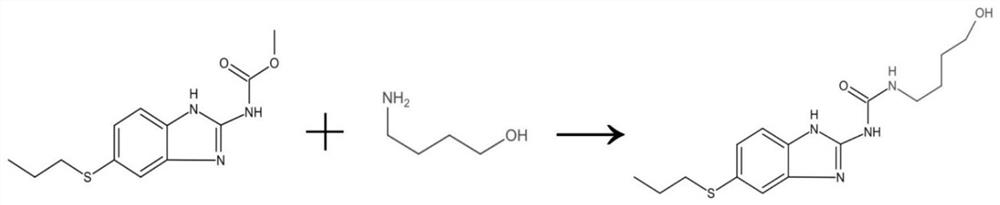
[0055] Weigh 6g of albendazole with an analytical balance, accurately measure 5mL of 4-amino-1-butanol and 27mL of N,N-dimethylformamide with a graduated cylinder, place the three in a round-bottomed flask, and stir evenly; The bottom flask was placed on a heated constant temperature magnetic stirrer, and reacted at 90°C for 24 hours. The reaction progress was detected by thin-layer chromatography, and compared with the albendazole raw material of the control group, it was determined that an intermediate was formed. After the reaction, the reaction solution was slowly added to ice water, stirred at 50...
Embodiment 2
[0065] An albendazole-cholic acid derivative, made from raw materials comprising the following components and contents: 15g of albendazole; 15mL of crosslinking agent; 70mL of N,N-dimethylformamide; 35g of cholic acid; Dicyclohexylcarbodiimide 12g; 4-dimethylaminopyridine 1.6g.
[0066] The method for preparing albendazole-cholic acid derivatives is the same as in Example 1.
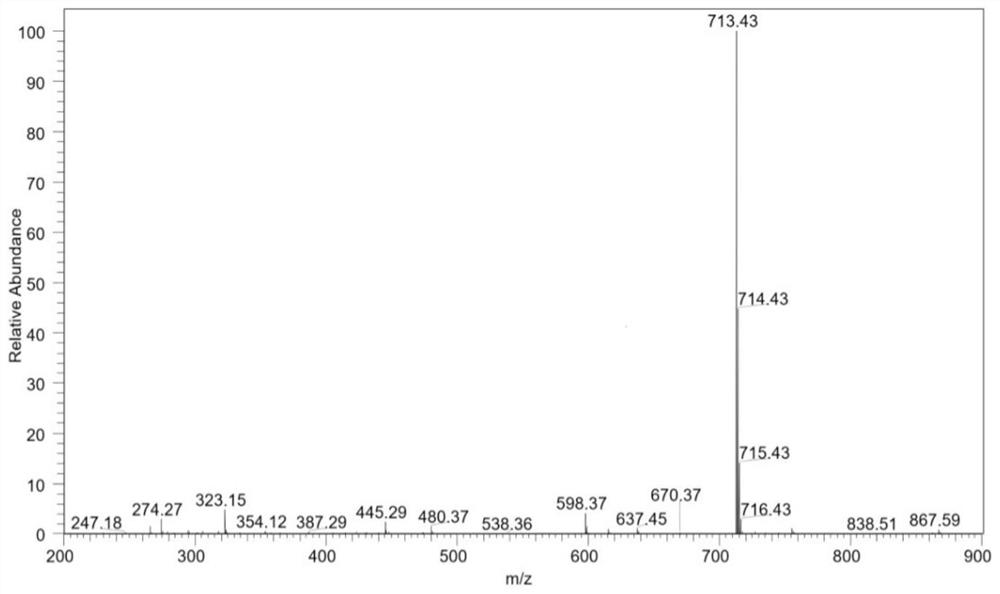
[0067] The mass of the finally obtained pure albendazole-cholic acid derivative was 496.6 mg.
Embodiment 3
[0069] An albendazole-cholic acid derivative is characterized in that it is made of raw materials comprising the following components and contents: 10g of albendazole; 10mL of crosslinking agent; 60mL of N,N-dimethylformamide; Cholic acid 27.2g; dicyclohexylcarbodiimide 10.5g; 4-dimethylaminopyridine 1.05g.
[0070] The method for preparing albendazole-cholic acid derivatives is the same as in Example 1.
[0071] The mass of the finally obtained pure albendazole-cholic acid derivative was 517.2 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com