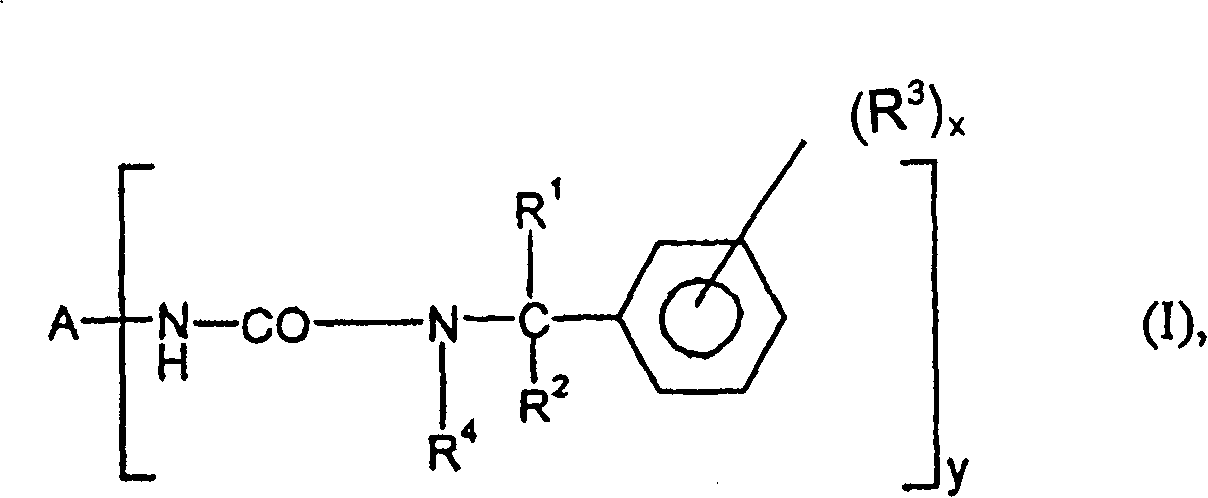
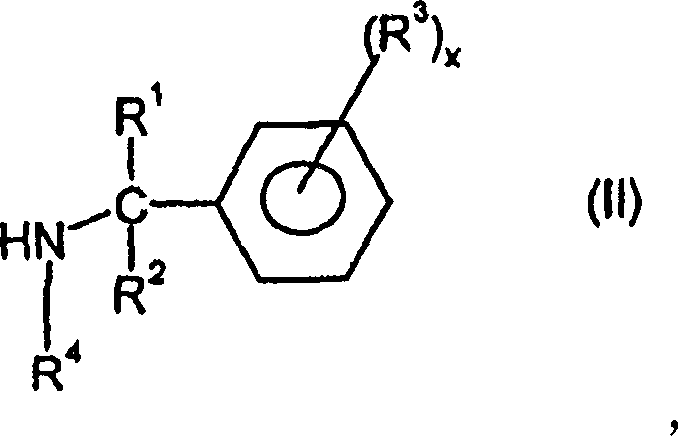
Dead-end polyisocyanic acid ester
A polyisocyanate and isocyanate-based technology, which is applied in the preparation of isocyanic acid derivatives, polyurea/polyurethane coatings, polyurea/polyurethane adhesives, etc., can solve the problems that coating properties can no longer be obtained
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] In the preparation of the polyisocyanates according to the invention, it is also possible to use catalysts, cosolvents and other auxiliaries and additives.
[0042] The blocked polyisocyanates of the invention are used as self-crosslinking one-component baking systems. They are added to formulations for the preparation of coatings, paints, inks and other baking systems such as binders for adhesives and elastomers, and as crosslinkers (components) for polyol components.
[0043] As noted above, the polyisocyanates of the present invention are either self-crosslinking polymers or can be used as crosslinking agents for the polyol component. Suitable polyol components which may also be used in admixture include the following:
[0044] Polyhydroxy polyesters, polyhydroxy polyethers, or addition polymers containing hydroxyl groups, examples are the polyhydroxy polyacrylates known per se. These compounds generally have a hydroxyl number of 20-200, preferably 50-130, based on...
Embodiment 1
[0055] Embodiment 1 (preparation of solvent-containing polyisocyanate crosslinking agent)
[0056] 117 g (0.6 equivalents) of 1,6-diisocyanatohexane (HDI) based 1,6-diisocyanatohexane (HDI) with an NCO content of 21.4 wt %, a viscosity of about 3000 mPa.s (23° C.) and a functionality of about 3.5 Polyisocyanates for industrial paints containing isocyanurates (Desmodur N3300, Bayer AG) was reacted with 98 g (0.6 equivalents) of benzyl tert-butylamine in 215 g of butyl acetate. The temperature rises to about 40°C. The reaction was complete within 2 hours. The blocked NCO value is 5.86%. The blocked isocyanates obtained in this way are used for the production of coating films.
[0057] Desmophen A 870 (Bayer AG), 70% BA solution 8.9g
[0058] The blocked polyisocyanate of embodiment 1, 50% BA solution 99.8g
[0059] Baysilone OL 17 (Bayer AG), 10% MPA solution 1.1 g
[0060] Modaflow (Solutia Inc.), 1% MPA solution 1.1 g
[0061] Tinuvin 292 (Ciba AG, Lamper...
Embodiment 2
[0077] Embodiment 2 (preparation of solvent-containing polyisocyanate crosslinking agent)
[0078]24.7 g (0.07 equivalents) of 1-isocyanato-3,5,5-trimethyl-5-isocyanate having an NCO content of 11.9 wt % and a viscosity (23° C.) of about 600 mPa.s Isocyanurate-containing polyisocyanates for industrial paints based on methylcarboxycyclohexane (isophorone diisocyanate, IPDI) (industrial product Desmodur Z4470, ex Bayer AG) was reacted with 11.4 g (0.07 equivalents) of benzyl tert-butylamine in 15.5 g of butyl acetate. The temperature rises to about 40°C. The reaction was complete within 2 hours. The blocked NCO value is 5.7%. The blocked isocyanates obtained in this way are used for the production of coating films.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com