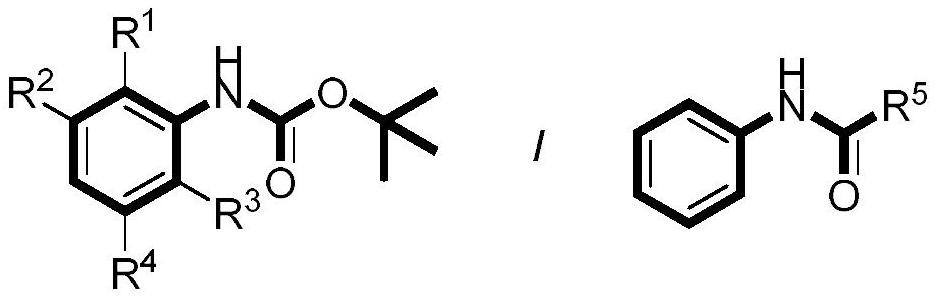
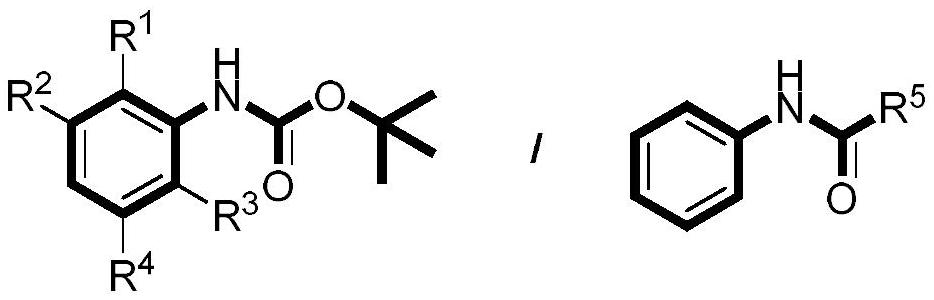
Preparation method of aniline para-trifluoromethylated derivative
A technology for trifluoromethylation and aniline derivatives is applied in the field of preparation of para-trifluoromethylated derivatives of aniline, and can solve the problems of low yield of stoichiometric metal salts, expensive reagents, narrow substrate range, and the like, To achieve the effect of easy availability of raw materials, simple post-processing process and various types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a preparation method of para-trifluoromethylated aniline derivatives, which comprises: sequentially adding aniline derivatives, 4,5-dichlorofluorescein, potassium persulfate, trifluoromethyl Put sodium sulfonate into a stirrer, use dimethyl sulfoxide as a solvent, and stir at 500 rpm for 11 hours at 23-25°C under 40W blue LED irradiation. After the reaction is completed, the product is subjected to column chromatography separation and purification treatment to obtain the para-trifluoromethylated aniline derivative.
[0027] The reaction process of above-mentioned technical scheme can be expressed as:
[0028]
Embodiment 1
[0032] This implementation case shows a preparation method of aniline para-trifluoromethylated derivatives according to the following steps: using N-Boc aniline as a raw material, the reaction formula is as follows:
[0033]
[0034] (1) Add 0.0386 grams (0.2 mmol) of N-Boc aniline, 0.0016 grams (4 mmol%) of 4,5-dichlorofluorescein, 0.1621 grams (0.6 mmol) of potassium persulfate, trifluoroform 0.0624 g (0.4 mmol) of sodium sulfinate and 2 ml of dimethyl sulfoxide were screwed on and placed between two 40w blue LEDs, and stirred at room temperature (23-25° C.) for 11 hours.
[0035] (2) Add water to quench the reaction after the reaction is completed, extract three times with ethyl acetate, combine the organic layers, and dry the solution with anhydrous sodium sulfate, spin the solvent under reduced pressure and separate by column chromatography (petroleum ether: ethyl acetate = 10:1) gave the product (73% yield).
[0036] 1 H NMR (400MHz, Chloroform-d) δ7.52(d, J=7.7Hz, ...
Embodiment 2
[0038] This implementation case shows a preparation method of aniline para-trifluoromethylated derivatives according to the following steps: using 2-ethyl-N-Boc aniline as a raw material, the reaction formula is as follows:
[0039]
[0040] (1) Add 0.0442 grams (0.2 mmol) of 2-ethyl-N-Boc aniline, 0.0016 grams (4 mmol%) of 4,5-dichlorofluorescein, and 0.1621 grams (0.6 mmol %) of potassium persulfate to a colorless glass bottle successively. ), 0.0624 g (0.4 mmol) of sodium trifluoromethanesulfinate and 2 ml of dimethyl sulfoxide, screw the lid on and place between two 40w blue LEDs, stir at room temperature (23-25°C) for 11 Hours.
[0041] (2) Add water to quench the reaction after the reaction is completed, extract three times with ethyl acetate, combine the organic layers, and dry the solution with anhydrous sodium sulfate, spin the solvent under reduced pressure and separate by column chromatography (petroleum ether: ethyl acetate = 10:1) gave the product (67% yield)....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com