Large-band-gap acceptor material based on fluorene or indenofluorene and thiophene end group, preparation method and application
A technology of indenofluorene and thiophene, which is applied in the field of organic solar cell material preparation, can solve the problems of not being suitable for indoor photovoltaic devices and narrow band gap, and achieve the effects of easy processing into film, large band gap and high mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Compound Synthesis:
[0068] The synthesis of compound 3 is mainly prepared by Knoevenagel condensation reaction of dialdehyde structure compound and terminal group compound, and its synthetic route is as follows:
[0069]
[0070] The specific operation is:
[0071] Add the above-mentioned dialdehyde structure compound 1 (0.20 g), terminal group 2 (0.21 g), 40 ml of dry chloroform and pyridine (1 ml) into a 100 ml two-necked bottle, react at room temperature for 24 hours, and use trichloro Extracted with methane, combined the organic phases, dried over anhydrous sodium sulfate, removed the solvent by rotary evaporation under reduced pressure, and separated by column chromatography to obtain compound 6 as a blue-black solid (0.25 g, yield 85%). 1 HNMR (400MHz, CDCl 3 )δ8.72(s,2H),7.85(s,2H),7.68(d,J=4.6Hz,4H), 7.59(s,2H),2.13-1.90(m,12H),1.10(d,J =25.8Hz,66H),0.83-0.65(m,24H ). 13 CNMR (101MHz, CDCl 3 ( s), 139.13(s), 137.53(s), 136.73(d, J=30.3Hz), 136.57-136....
Embodiment 2
[0073] The test of the ultraviolet-visible absorption spectrum of organic optoelectronic compound 3:
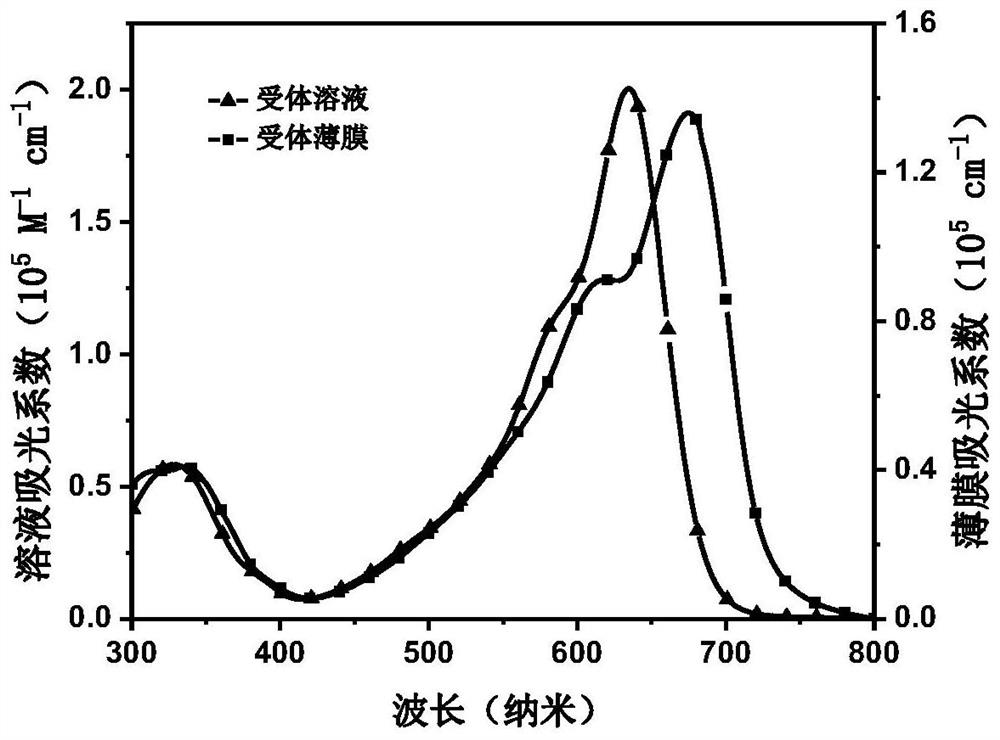
[0074] The compound prepared by a kind of embodiment is made into 10 -5 mol / L and 10 -2 mol / L chloroform solution, the former is used to measure the UV-visible absorption spectrum in the solution state, and the latter solution is used to measure the UV-visible absorption spectrum of the film at a speed of 1200 rpm on a quartz plate, and the scanning range is 300-800 nanometers, the measuring instrument is PerkinElmer Lambda 20UV / VIS Spectrophotometer.
[0075] The UV-Vis absorption spectrum of compound 3 is as figure 1 shown. The solution of this compound has stronger absorption in the range of 400-700 nanometers, and the maximum absorption peak position is at 640 nanometers, while the film absorption has obvious red shift and the absorption range becomes wider, and the maximum absorption peak is at 690 nanometers, which is the same as High photoelectric conversion effici...
Embodiment 3
[0078] Comparative compound 3, preparation and testing of indoor and outdoor organic solar cell devices using the compound of formula (1) of the present application as electron acceptor:
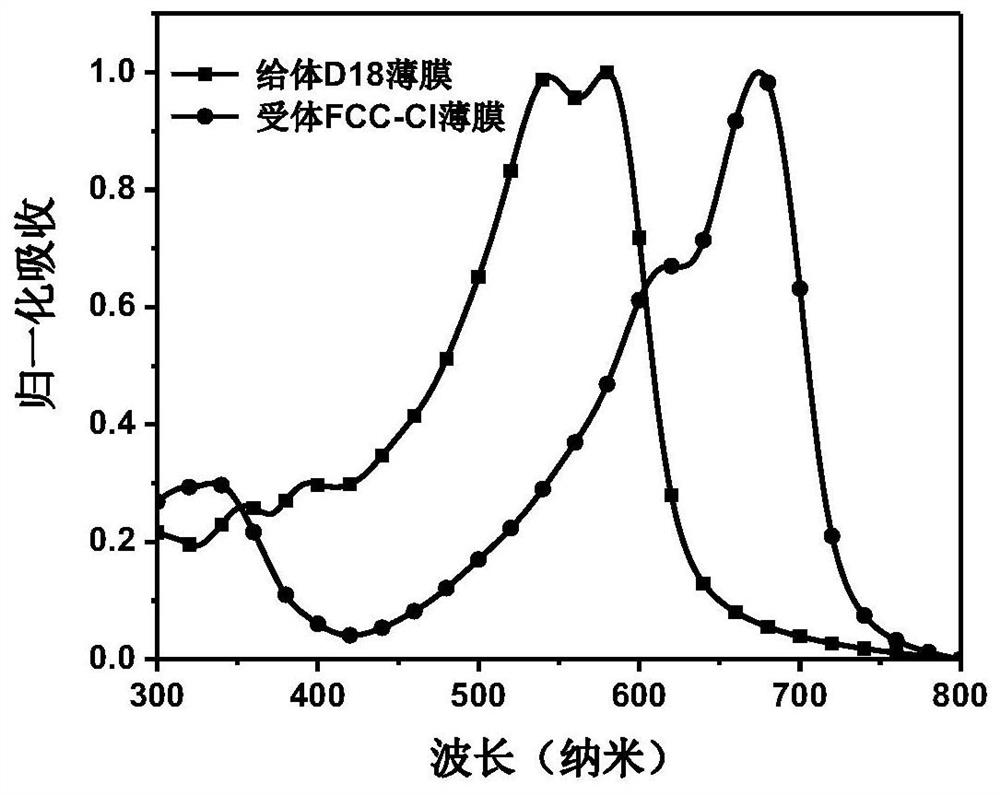
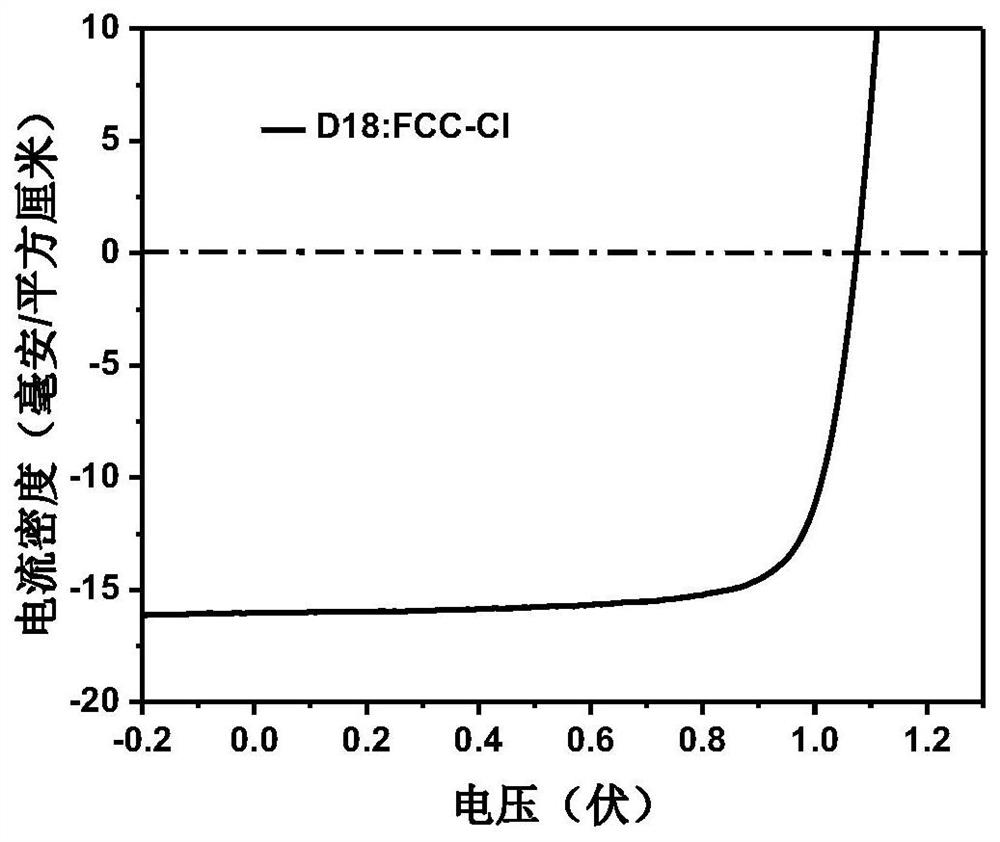
[0079] The photovoltaic device structure is ITO / PEDOT:PSS / donor material:acceptor material / PDI-NO / Al. The specific preparation process of photovoltaic devices is: ITO (indium tin oxide, anode) glass pretreatment: first scrub the ITO glass with detergent, then rinse it with deionized water, and then use acetone and isopropanol solvents to ultrasonically clean each 20 After taking it out, blow it dry with a nitrogen gun, and treat it with UV-ozone for 20 minutes. Spin-coat one deck of PEDOT:PSS (CleviosPVPAl4083) on the ITO glass after pretreatment as the anode interface layer, treat that PEDOT:PSS bakes in oven at 140 ℃ for 20 minutes, after cooling, compound 6 prepared in Example 1 and donor material The blended chloroform solution was spin-coated on the surface of PEDOT:PSS as an active la...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com