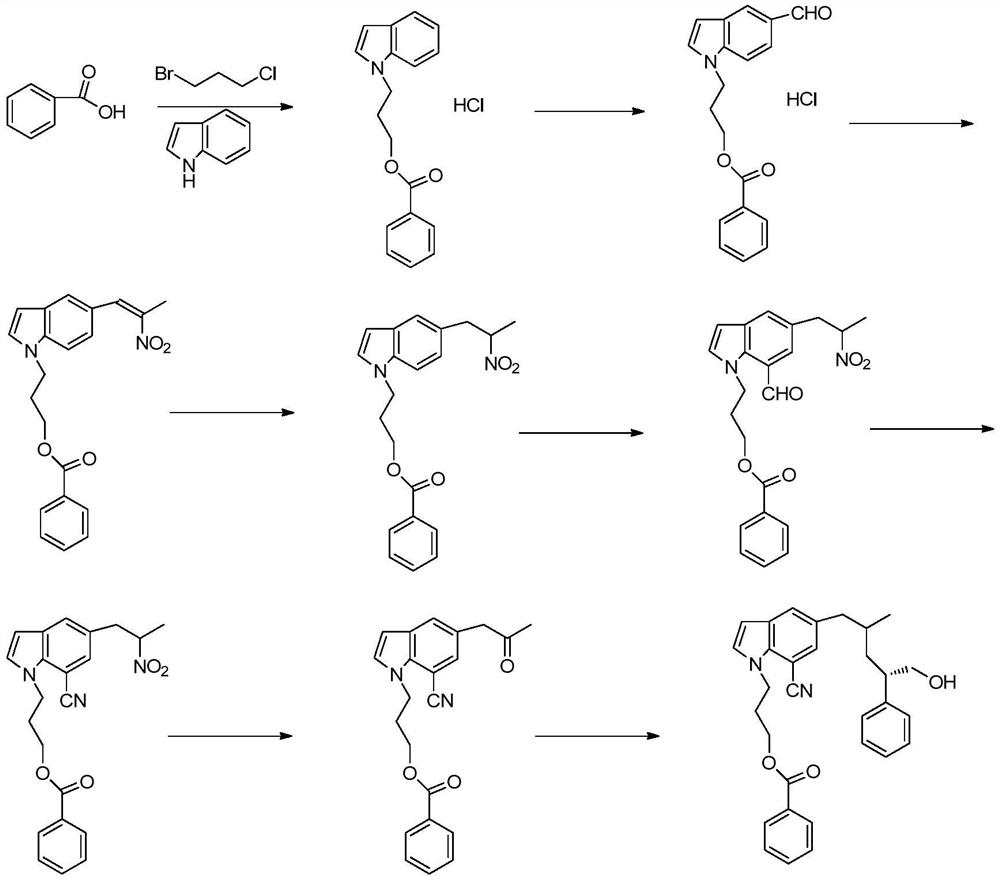
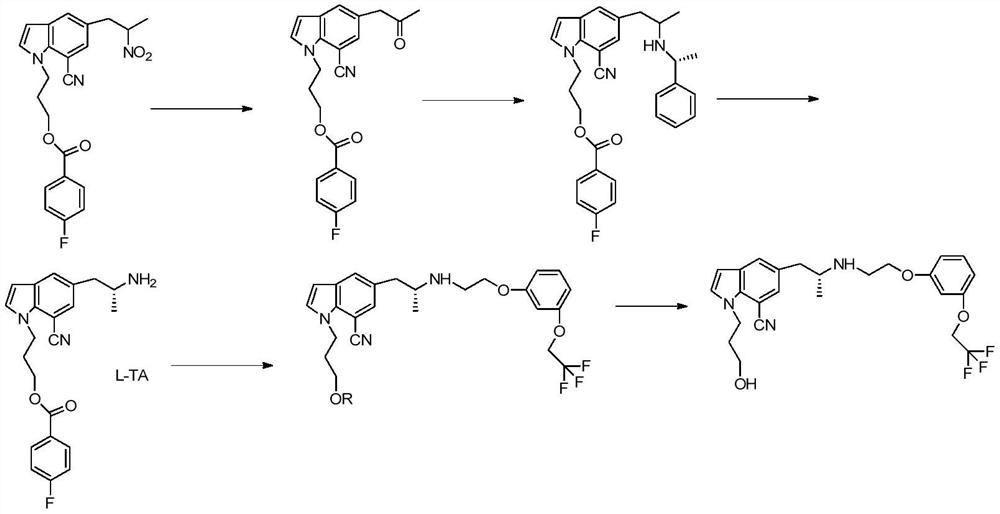
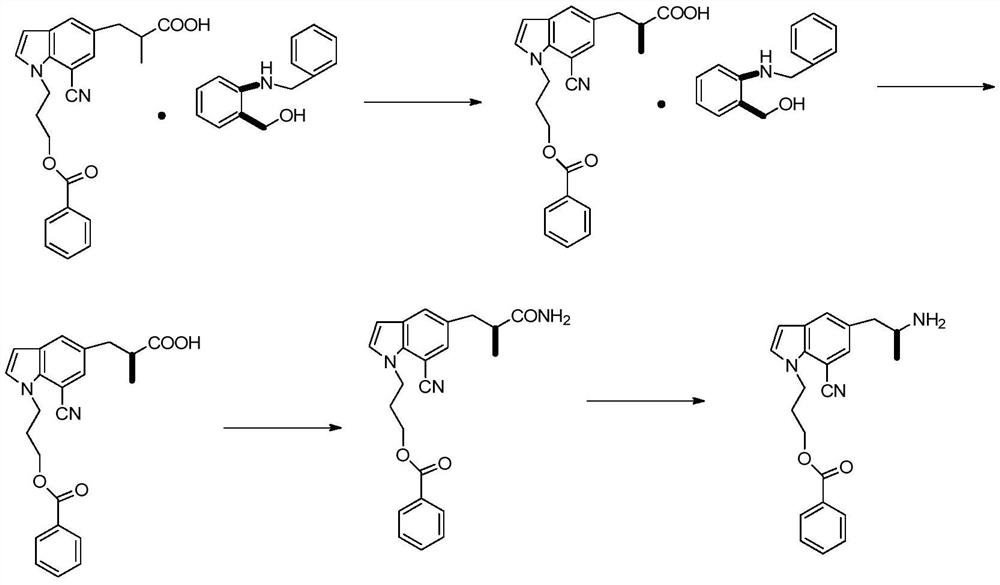
A kind of synthetic method of silodosin
A synthesis method and technology of silodosin are applied in the field of preparation of silodosin, a drug for treating benign prostatic hyperplasia, can solve the problems of difficult reaction operation, complicated preparation process, unfavorable industrial production and the like, and achieve easy industrial production and product purity. The effect of high and low labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1 general formula 2 is synthesized
[0081] Add 20g (0.168mol) of general formula 1 indoline into the flask, add dropwise 14.5g (0.185mol) acetyl chloride at 20°C, add 100g water to quench after the reaction is complete, then extract with 100g DCM (dichloromethane), and the DCM phase The solvent was removed under reduced pressure to obtain intermediate 1, 20ml of acetic acid was added and used directly for the next step; 26.8g (0.168mol) of Br was added dropwise at 20-25°C 2 Mix the solution with 20ml of acetic acid, react at 25-30°C for 3 hours, add 100g of water, add 100ml of DCM, separate the liquid, remove the solvent from the DCM phase under reduced pressure to obtain 38.68g of intermediate 2, Y=96%, directly used in the next step; Add 100g ETOH / water (2:1), add 20g concentrated hydrochloric acid, heat up to 78°C, react for 4 hours, cool down to room temperature after the reaction, remove ethanol under reduced pressure, extract three times with 50g DCM e...
Embodiment 2
[0083] Embodiment 2 general formula 3 is synthesized
[0084] Add compound 2 (5-bromoindoline) 30g (0.151mol), 23g (0.166mol) potassium carbonate, 12.6g (0.08mol) potassium iodide, 90gDMF to the flask, heat up to 90-100°C, add dropwise 29.8g ( 0.167mol) 2-(3-chloropropoxy)tetrahydro-2H-pyran Raise the temperature to 130°C and react for 6 hours. After the reaction is complete, add 200g of water, extract three times with 80g of ethyl acetate each time, remove the solvent from the organic phase under reduced pressure, and recrystallize with 110g of toluene to obtain 46.4g of 5-bromo-1-[2-tetra Hydropyran]ethyl}-2Hindole, Y=90%, HPLC purity 98%.
[0085] 1 H NMR (500MHz, CDCl 3 ): δ7.22-7.18(m,2H), 7.06(m,1H), 4.60(m,1H), 3.78-3.61(m,6H), 3.35(m,2H), 2.96(m,2H), 1.81-1.56 (m, 8H). MS: 339-340 (MH). HPLC: XDB-C184.6*250, acetonitrile / water 1:1, 230nm, 45min.
Embodiment 3
[0086] Embodiment 3 general formula 6 is synthesized
[0087] Add 35g (0.393mol) D-alanine (compound 4), 33g (0.825mol) sodium hydroxide, 150g water to the flask, add 1.05 equivalents of 70.4g (0.41mol) CbzCl (benzyl chloroformate) dropwise at 10-15°C Esters), raised to 20-30°C after adding, reacted for 5 hours, after the reaction, adjusted the pH to 2 with 3M hydrochloric acid, extracted three times with 100g toluene each time, removed the solvent under reduced pressure to obtain the general formula 5, which was directly used in the next step Reaction; then add 180g toluene, 1.1 equivalents of 74.4g (0.432mol) p-toluenesulfonic acid and 11.8g (0.393mol) formaldehyde to the general formula 5, start stirring, and react at 20-25°C for 12h. After the reaction, the toluene phase is washed with water Twice, the toluene phase was collected, and the solvent was removed under reduced pressure to obtain 86.9 g of the compound of general formula 6, Y=94%, HPLC purity 97%.
[0088] 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com