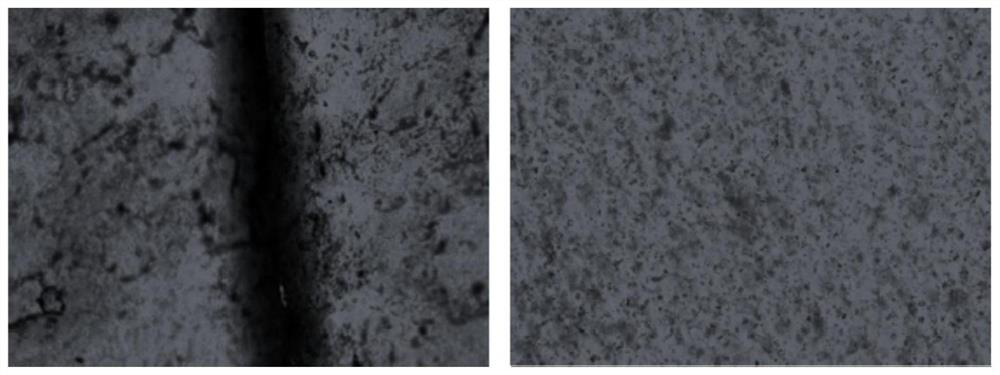
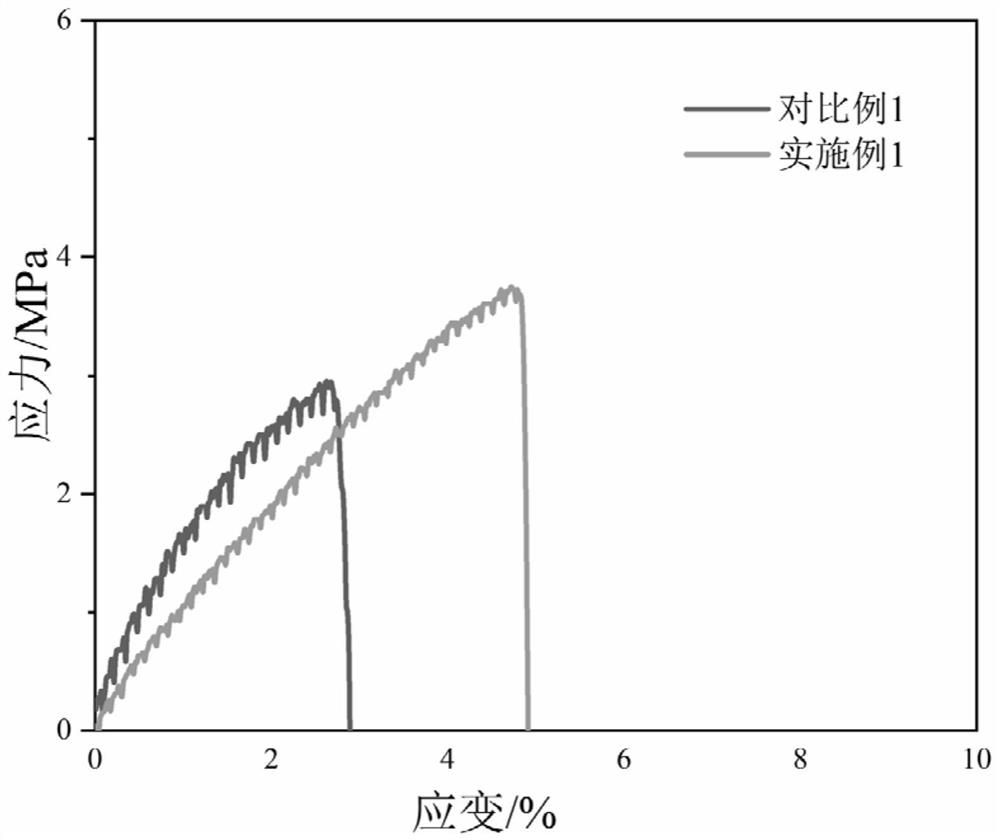
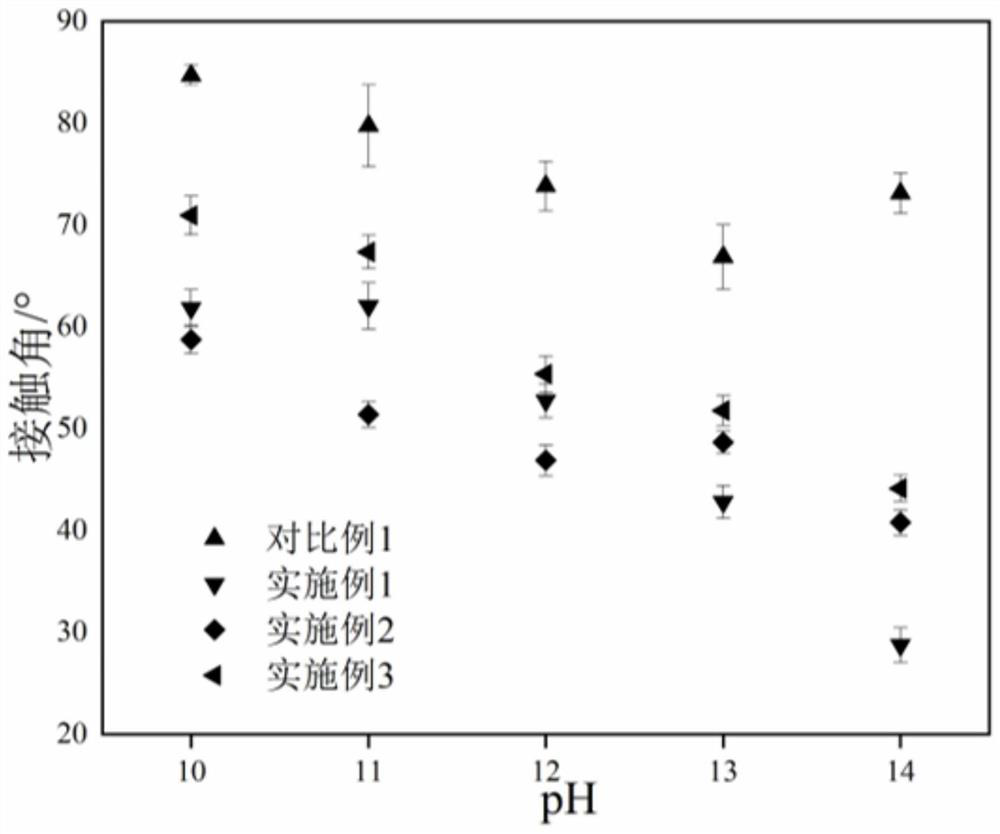
A kind of polyurethane with pH response and self-healing properties and preparation method thereof
A self-healing, polyurethane material technology, applied in the field of polymer materials to achieve the effect of improving damage recovery and improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The present embodiment adopts the following raw materials:
[0041] 20g polycaprolactone diol (Mn~2000)
[0042] 160mL Tetrahydrofuran (THF)
[0043] 0.04g Dibutyltin dilaurate (DBTDL)
[0044] 0.856g Bis(2-hydroxyethyl)disulfide (SS)
[0045] 0.7408g 2,2-Dimethylolbutyric acid (DMBA)
[0046] 3.383g hexamethylene diisocyanate (HDI)
[0047] The preparation method of the present embodiment is:
[0048]In a three-necked flask equipped with an electric stirrer, a condenser tube and a vacuum tailpiece, polycaprolactone diol was added, and after vacuum dehydration at 110-120 °C for 2 hours, the temperature was lowered to about 60 °C, and HDI and an appropriate amount of tetrahydrofuran were added. Under nitrogen protection, the reaction was stirred at 60° C. for 1 hour to obtain a prepolymer. Add 0.856g bis(2-hydroxyethyl) disulfide (SS) and 0.7408g 2,2-dimethylolbutyric acid (DMBA) to the prepolymer to continue the reaction until the isocyanate is consumed completely...
Embodiment 2
[0073] The present embodiment adopts the following raw materials:
[0074] 10g polycaprolactone diol (Mn~1000)
[0075] 160mL N,N-Dimethylformamide (DMF)
[0076] 0.04g Dibutyltin dilaurate (DBTDL)
[0077] 0.8569g Bis(2-hydroxyethyl)disulfide (SS)
[0078] 0.7408g 2,2-Dimethylolbutyric acid (DMBA)
[0079] 3.3837g hexamethylene diisocyanate (HDI)
[0080] The preparation method of the present embodiment is:
[0081] In a three-necked flask equipped with an electric stirrer, a condenser tube and a vacuum tailpiece, polycaprolactone diol was added, and after vacuum dehydration at 110-120°C for 2 hours, the temperature was lowered to about 60°C, and HDI and an appropriate amount of N,N were added. -Dimethylformamide, stirred and reacted at 60°C for 1 hour under nitrogen protection to obtain a prepolymer. Add 0.856g bis(2-hydroxyethyl) disulfide (SS) and 0.7408g 2,2-dimethylolbutyric acid (DMBA) to the prepolymer to continue the reaction until the isocyanate is completely c...
Embodiment 3
[0097] The present embodiment adopts the following raw materials:
[0098] 10.6g polycaprolactone diol (Mn~530)
[0099] 160mL N,N-Dimethylformamide (DMF)
[0100] 0.04g Dibutyltin dilaurate (DBTDL)
[0101] 1.7138g Bis(2-hydroxyethyl)disulfide (SS)
[0102] 1.4966g 2,2-Dimethylolbutyric acid (DMBA)
[0103] 6.9654g hexamethylene diisocyanate (HDI)
[0104] The preparation method of the present embodiment is:
[0105] In a three-necked flask equipped with an electric stirrer, a condenser tube and a vacuum tailpiece, polycaprolactone diol was added, and after vacuum dehydration at 110-120°C for 2 hours, the temperature was lowered to about 60°C, and HDI and an appropriate amount of N,N were added. - Dimethylformamide, stirred and reacted at 60° C. for 1 hour under nitrogen protection to obtain a prepolymer. Add 0.856g bis(2-hydroxyethyl) disulfide (SS) and 0.7408g 2,2-dimethylolbutyric acid (DMBA) to the prepolymer to continue the reaction until the isocyanate is complete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com