Synthesis method of intramolecular exciton splitting material with anti-aromaticity and quinonoid structure, preparation method of thin film and single crystal
A synthetic method and aromatic technology, which is applied in the synthesis, thin film preparation and single crystal preparation of intramolecular exciton splitting materials with antiaromaticity and quinone structure, and can solve the problems of chromophore sensitivity and limited development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A method for synthesizing a material with intramolecular exciton splitting properties, comprising the following steps:
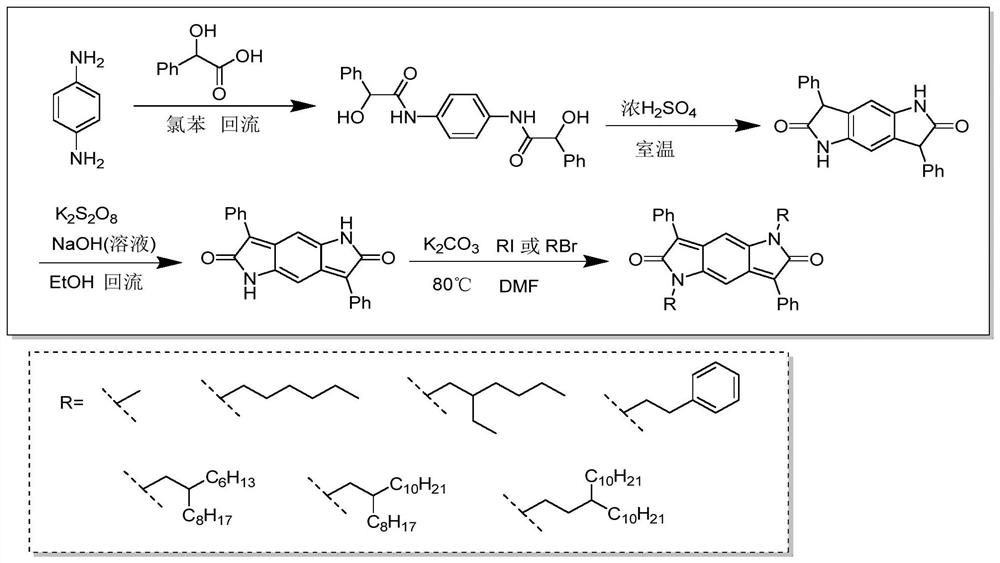
[0028] Synthesize diketopyrrolopyrrole (BDPP) as the molecular skeleton and introduce different alkyl chain substituents on the nitrogen atom of the amide bond.
[0029] For example, 1,5-dihexyl-3,7-diphenylpyrrolopyrrole-2,6-dione (designated BDPP-C6) was synthesized. The BDPP-C6 molecule is taken as an example for illustration below.
[0030] A method for preparing a thin film of a material with an intramolecular exciton splitting property, comprising synthesizing the above molecule and preparing a thin film of the molecule by vacuum vapor deposition.
[0031] For example, to prepare BDPP-C6 molecules, a thin film of BDPP-C6 molecules is prepared by vacuum vapor deposition.
[0032] A method for preparing a single crystal of an intramolecular exciton splitting material, comprising synthesizing the above molecules: dissolving a small amount of mole...
Embodiment 1
[0039] Embodiment one: if figure 1 The synthesis route of the BDPP skeleton shown is illustrated by taking BDPP-C6 as an example.
[0040] Synthesis of BDPP-C6 molecule: ①Add p-phenylenediamine and mandelic acid to chlorobenzene, react at a certain temperature (for example, 130°C) for several hours, such as 18 hours, cool to room temperature, filter the precipitate, and wash with ethanol , dried to obtain a white solid BP molecule (C 22 h 20 N 2 o 4 ). 2. Add BP molecules into concentrated sulfuric acid, stir at room temperature for example for 20 hours, pour into ice water after the reaction is over, filter the precipitate, wash with water, and dry to obtain light red solid BDP molecules (C 22 h 16 N 2 o 2 ). ③ Add sodium hydroxide solution dropwise to the ethanol suspension of BDP molecules, then add aqueous potassium persulfate solution, heat and reflux at, for example, 78°C for 2 hours, and after cooling, filter the precipitate, wash with water, and dry to obtain ...
Embodiment 2
[0042] Example 2: Preparation and characterization of BDPP-C6 molecular film:
[0043] Preparation of BDPP-C6 molecular film: at 1×10 -5 mbar vacuum condition, to BDPP-C6 molecular film was prepared by vacuum vapor deposition method on sapphire or quartz substrate, and the evaporated molecular film was 100-120nm.
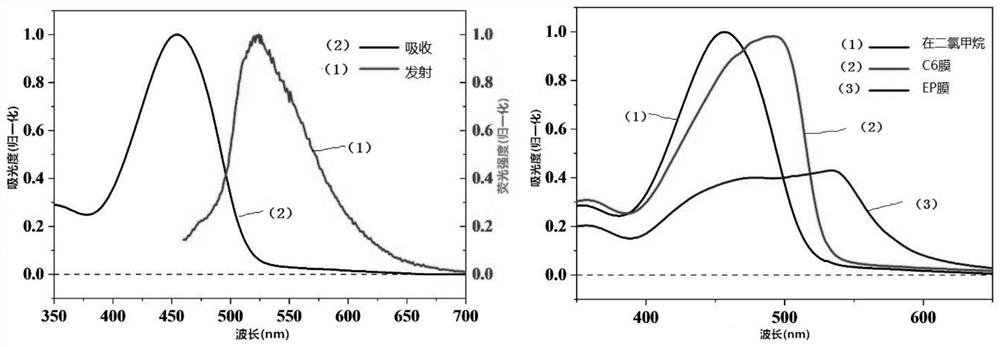
[0044] figure 2 Shown are the absorption emission spectrum of BDPP-C6 solution and the absorption spectrum of film. The maximum absorption peak of BDPP-C6 molecule in solution is at the wavelength of 456nm, and the highest emission peak is at the wavelength of 532nm. The maximum absorption peak of the BDPP-C6 molecular film has an obvious red shift compared with the solution, the absorption band edge is at 530nm, and the band gap is about 2.4eV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com