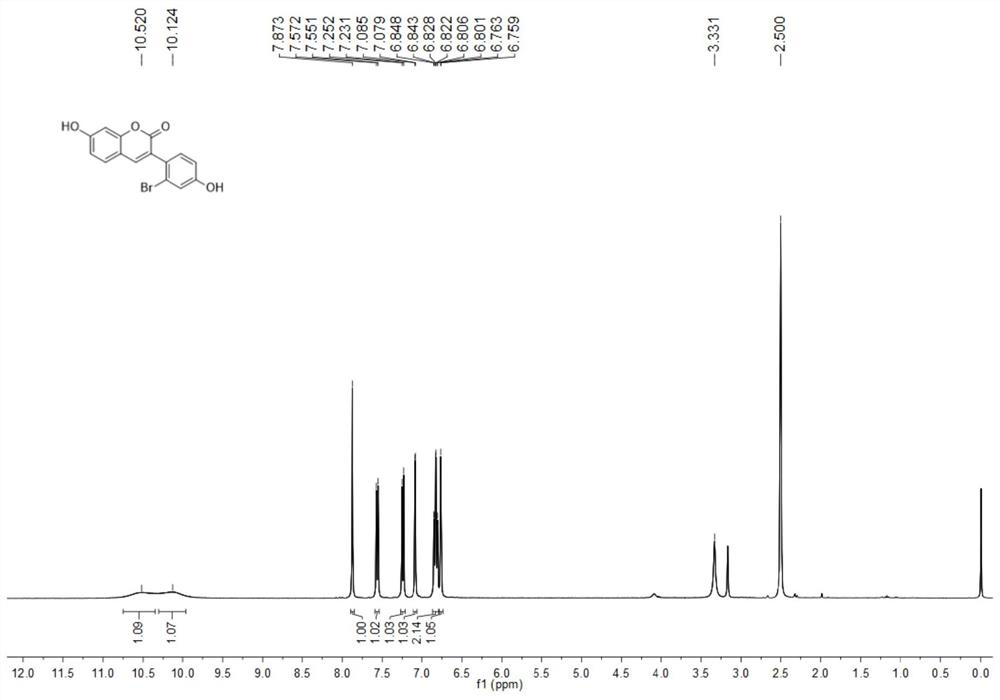
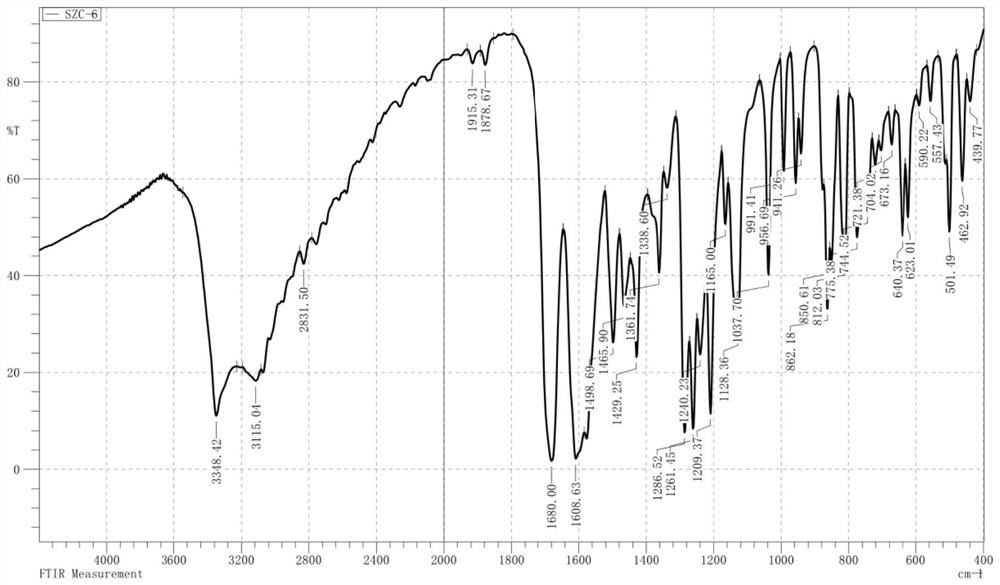
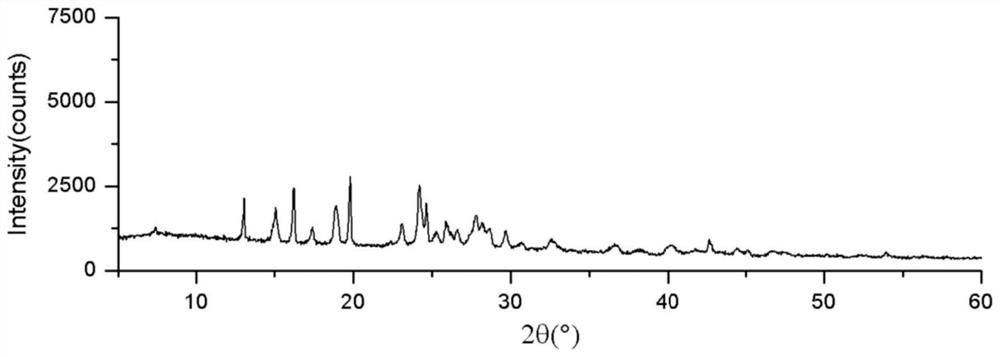
3-aryl coumarin derivative as well as preparation method and application thereof
A technology of aryl coumarin and hydroxy coumarin, which is applied in the directions of drug combination, pharmaceutical formulation, medical preparation containing active ingredients, etc., can solve the problems of low bioavailability, poor pharmacokinetics, poor specificity, etc. , to achieve the effect of obvious specificity, good affinity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: the preparation of 3-(2-hydroxyphenyl)-6-methylcoumarin (1)
[0075] 2-Hydroxyphenylacetic acid (5 g, 32.86 mmol), 2-hydroxy 5-methylbenzaldehyde (4.47 g, 32.86 mmol), acetic anhydride (20.13 g, 197.18 mmol) and triethylamine (13.30 mmol) were added to a round bottom flask. g, 131.45mmol), stirred and reacted at 120°C for 8h, and monitored the progress of the reaction by thin-layer chromatography. After the reaction is completed, according to post-processing method B, pour the reaction solution into ice water and keep stirring to obtain a viscous solid, pour out the supernatant, soak and wash the viscous material in water until the water phase is close to neutral. Add 50 mL of sodium hydroxide (2.63 g, 65.73 mmol) aqueous solution to the above viscous material, stir to dissolve, extract and wash with ethyl acetate twice, separate the liquids to obtain an aqueous layer, slowly add dilute hydrochloric acid aqueous solution dropwise to adjust pH = 2, A large...
Embodiment 2
[0076] Embodiment 2: the preparation of 3-(2-hydroxyphenyl)-6-methylcoumarin (1)
[0077] Into a round bottom flask was added 2-hydroxyphenylacetic acid (5 g, 32.86 mmol), 2-hydroxy 5-methylbenzaldehyde (4.47 g, 32.86 mmol), acetic anhydride (16.77 g, 164.31 mmol) and triethylamine (9.98 g, 98.59mmol), stirred and reacted at 110°C for 10h, and monitored the progress of the reaction by thin-layer chromatography. After the reaction is completed, according to post-processing method B, pour the reaction solution into ice water and keep stirring to obtain a viscous solid, pour out the supernatant, soak and wash the viscous material in water until the water phase is close to neutral. Add 50 mL of sodium hydroxide (2.63 g, 65.73 mmol) aqueous solution to the above viscous material, stir to dissolve, extract and wash with ethyl acetate twice, separate the liquids to obtain the water layer, slowly add dilute hydrochloric acid aqueous solution dropwise, and adjust pH=3 , a large amount...
Embodiment 3
[0078] Example 3: Preparation of 3-(2-hydroxyl-4-methoxyphenyl)-6-chlorocoumarin (2)
[0079] To a round bottom flask was added 2-hydroxy-5-methoxyphenylacetic acid (5 g, 27.45 mmol), 2-hydroxy-4-chlorobenzaldehyde (4.30 g, 27.45 mmol), acetic anhydride (16.81 g, 164.68 mmol) and triethylamine (11.11g, 109.78mmol), stirred and reacted at 120°C for 8h, and monitored the progress of the reaction by thin-layer chromatography. After the reaction is completed, according to post-processing method B, pour the reaction solution into ice water and keep stirring to obtain a viscous solid, pour out the supernatant, soak and wash the viscous material in water until the water phase is close to neutral. Add 50mL of sodium hydroxide (2.20g, 54.89mmol) aqueous solution to the above viscous material, stir to dissolve it, extract and wash with ethyl acetate twice, separate the liquids to obtain an aqueous layer, slowly add dilute hydrochloric acid aqueous solution dropwise, and adjust pH=2 , a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com