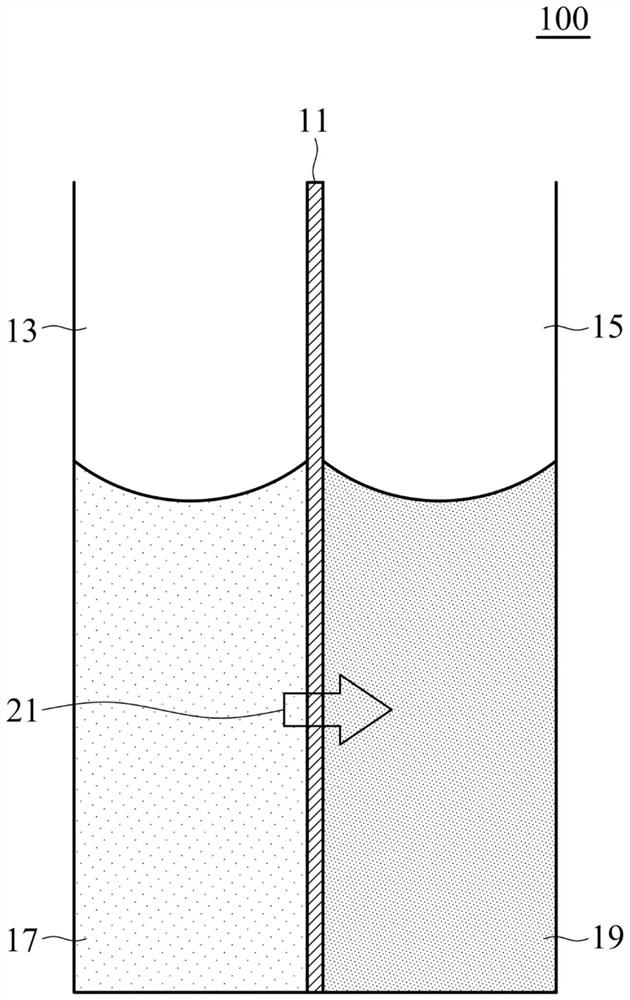
Ionic liquid and forward osmosis process using same
An ionic liquid, forward osmosis technology, applied in the field of forward osmosis process, can solve the problems of high energy consumption and toxicity, and achieve the effects of low energy consumption, high hydrophilicity, and low film blocking rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] First, synthesize (2-hydroxyethyl) octyl diethylammonium bromide ((2-hydroxyethyl) octyldiethylammonium bromide according to the following steps, the structure is ) (hereinafter referred to as [Ch228][Br]):
[0047] Add 52 grams of (2-hydroxyethyl) diethylamine (0.44 mole), 85 grams of (1-octylbromide) (0.44 mole), and 150 milliliters of acetonitrile into a reaction flask, And the resulting mixture was stirred at 80°C for 24 hours. After cooling down to room temperature, the obtained product was slowly dropped into 1.5 L of diethylether, and a white solid was observed to precipitate. After filtering, the obtained filter cake is dried to obtain (2-hydroxyethyl)octyldiethylammonium bromide ([Ch228][Br]).
[0048] Next, [Ch228][Br] is converted into (2-hydroxyethyl)octyldiethyl ammonium hydroxide (2-hydroxyethyl)octyldiethyl ammonium hydroxide by ion exchange resin, the structure is ) (hereinafter referred to as [Ch228][OH]). Then, 82.8 grams of [Ch228][OH] (0.335mol...
Embodiment 2
[0057] First, 1,8-octanediyl-bis((2-hydroxyethyl)diethylammonium bromide (1,8-octanediyl-bis((2-hydroxyethyl)diethylammonium)dibromide) was synthesized according to the following steps, the structure for ) (hereinafter referred to as [DCh8-22] [Br 2 ]):
[0058] 52 grams of (2-hydroxyethyl) diethylamine ((2-hydroxyethyl) diethylamine) (0.44mole), 60 grams of 1,8-dibromooctane (1,8-dibromooctane) (0.22mole), and 100 mL of acetonitrile was added to a reaction flask, and the resulting mixture was stirred at 80° C. for 24 hours. After cooling down to room temperature, the obtained product was slowly dropped into 1.5 L of diethyl ether, and a white solid was observed to precipitate. After filtering, the resulting filter cake is dried to obtain 1,8-octanediyl-bis((2-hydroxyethyl) diethylammonium bromide ([DCh8-22][Br 2 ]).
[0059] Next, [DCh8-22][Br 2 ] into (2-hydroxyethyl)octyldiethyl ammonium hydroxide ((2-hydroxyethyl)octyldiethyl ammonium hydroxide, the structure is )...
Embodiment 3
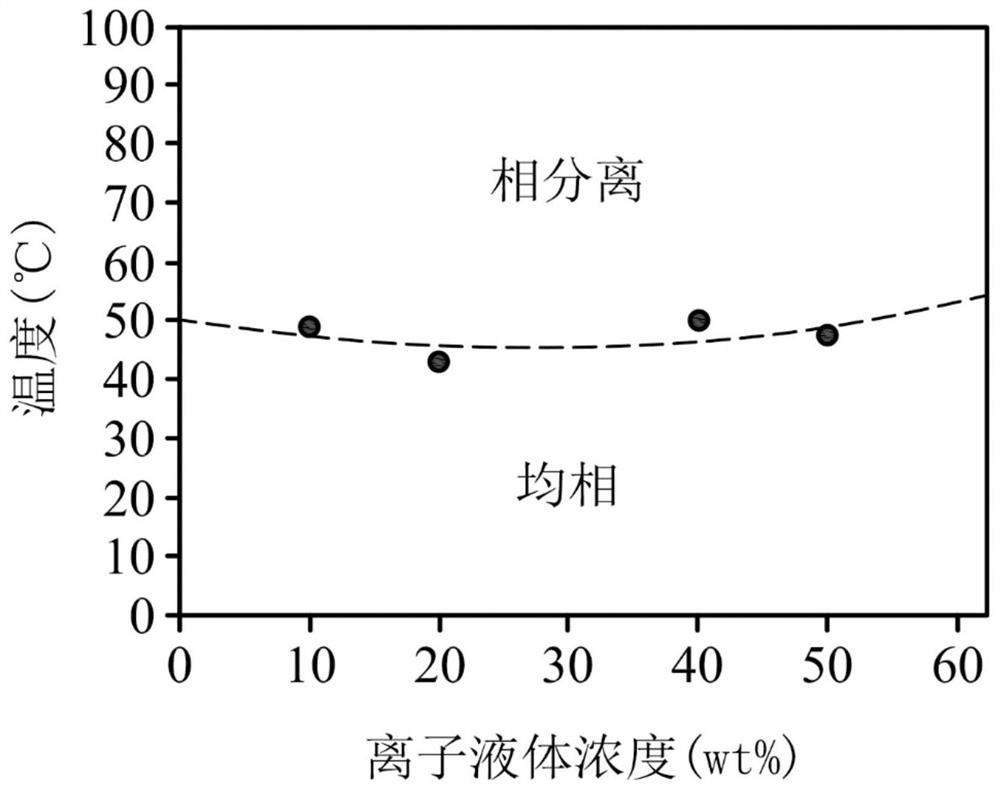
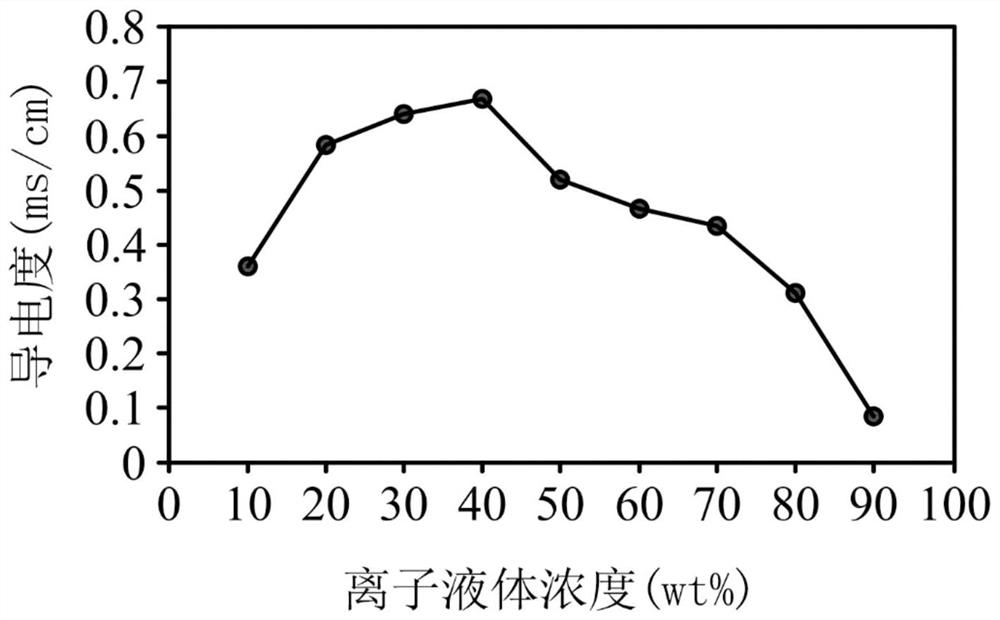
[0064] Add 100 grams of choline hydroxide aqueous solution (46 wt%, dissolved in water) (0.38 mole of choline) into a reaction flask. Next, 74.42 grams of azelaic acid (nonanedioic acid) was slowly added into the reaction flask. After reacting at room temperature for 24 hours, the resulting solution was extracted with 200 ml of dichloromethane, and the organic layer was collected. After removing water and concentrating, obtain product ionic liquid (III) (structure is Hereinafter referred to as [Ch][Aze]. [Ch][Aze] was analyzed by NMR spectroscopy, the results are as follows: 1 H-NMR (500MHz in D 2 O):1.17(m,6H,-CH 2 -), 1.41(m,4H,-CH 2 -), 2.10(t,4H, - OOCCH 2 -), 3.03(s,9H,N + CH 3 ), 3.35(t,2H,N + CH 2 CH 2 -), 3.90(m,2H,-CH 2 OH).
[0065] After mixing the ionic liquid [Ch][Aze] with water in different weight ratios, let it stand at room temperature for a period of time, observe whether phase separation occurs, and record its critical solution temperature, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com