Preparation method of clindamycin phosphate freeze-dried powder injection for injection
A technology of clindamycin phosphate and freeze-dried powder injection, which is applied in the field of preparation of clindamycin phosphate freeze-dried powder injection to achieve the effect of avoiding the precipitation of impurities, avoiding decomposition and reducing hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 A kind of preparation method of clindamycin phosphate freeze-dried powder for injection
[0038] This embodiment is a preparation method of clindamycin phosphate freeze-dried powder for injection. The specific preparation process includes the following steps carried out in sequence:
[0039] 1) refining of clindamycin phosphate
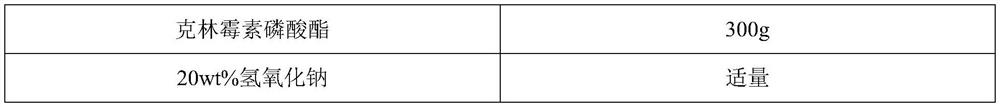
[0040] Take 5L of methanol aqueous solution with a temperature of 20°C and a methanol content of 71.5vol%, add 1 kg of crude clindamycin phosphate [purity 98.10%, according to "Chinese Pharmacopoeia" (2015 edition of four general rules 0512), using high performance liquid chromatography Determination], stir and fully disperse in methanol aqueous solution, slowly add 20wt% sodium hydroxide aqueous solution dropwise at a rate of 0.5L / h, keep the system temperature at 20°C during the dropping process, and stir to fully dissolve clindamycin phosphate After the crude ester, add aqueous sodium hydroxide solution dropwise until the crude...
Embodiment 2~6
[0060] Example 2-6 The preparation method of clindamycin phosphate freeze-dried powder for injection
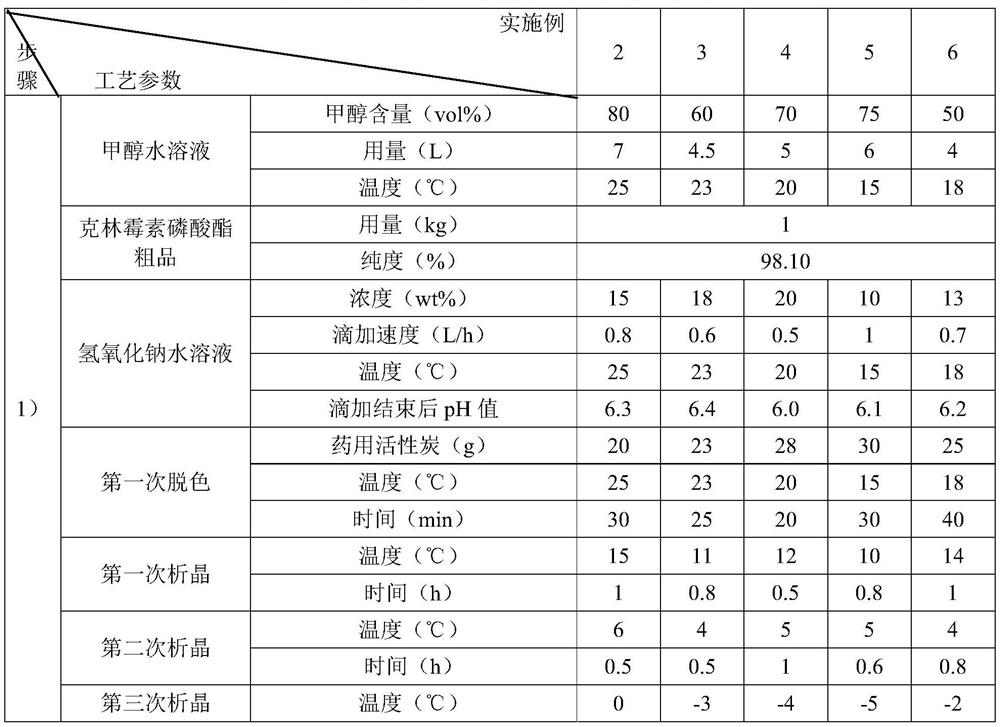
[0061] Examples 2 to 6 are respectively a preparation method of clindamycin phosphate freeze-dried powder for injection, and their steps are basically the same as those in Example 1, except that the amount of raw materials and process parameters are different. See Table 3:
[0062] List of various technological parameters in table 3 embodiment 2~6
[0063]
[0064]
[0065]
[0066] The content of the other parts of Examples 2-6 is the same as that of Example 1.
[0067] The clindamycin phosphate freeze-dried powder for injection prepared in Examples 1-6 has good stability and clarity, high main drug content, less impurities, smooth surface, good appearance and excellent resolubility.
experiment example 1
[0068] Influencing factors in experimental example 1 clindamycin phosphate refining method
[0069] Using the same batch of clindamycin phosphate crude product (purity 98.10%), comparative examples 1~10 and the comparative test of clindamycin phosphate refining process in embodiment 1, the difference is only in:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com