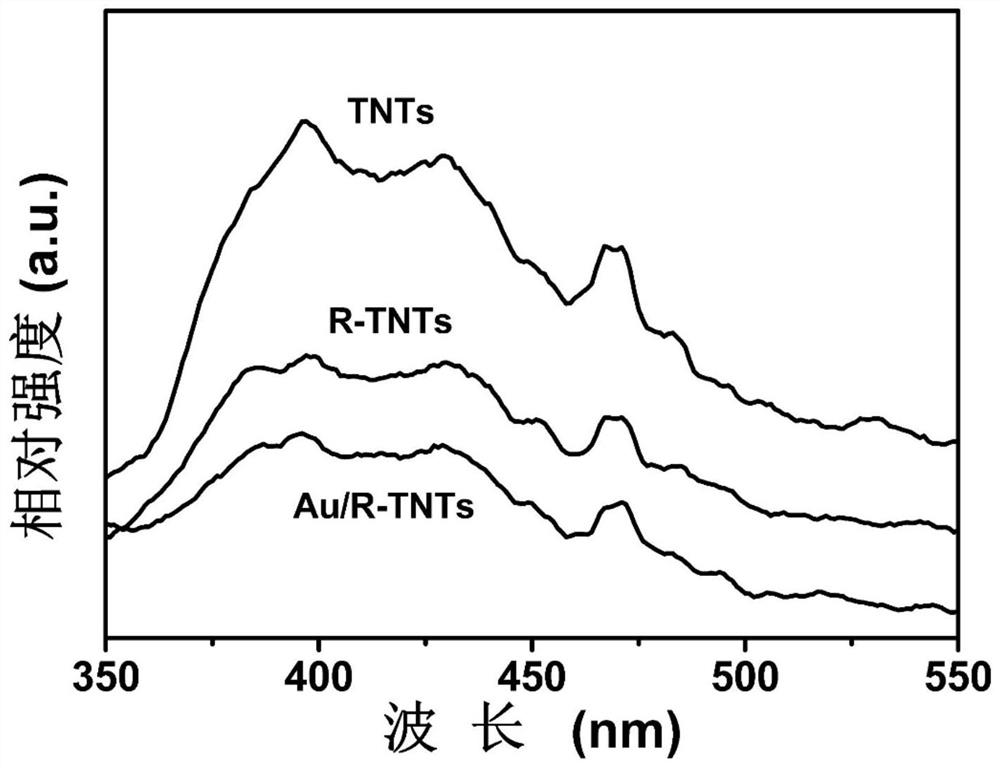
High-activity electrochemical self-doped TiO2 nanotube-based material as well as preparation and application thereof
A nanotube and self-doping technology, which is applied in nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve the problems of reducing CO2 output and low light utilization rate, slow reaction rate, and high activation energy. , to achieve the effect of improving electrical conductivity, easy separation, and catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A highly active electrochemical self-doped TiO 2 Preparation of Nanotube-Based Materials and Its Gas-Phase Photoelectrocatalytic Reduction of CO 2 method, including the following steps:
[0041] (1) Preparation of TiO 2 nanotube
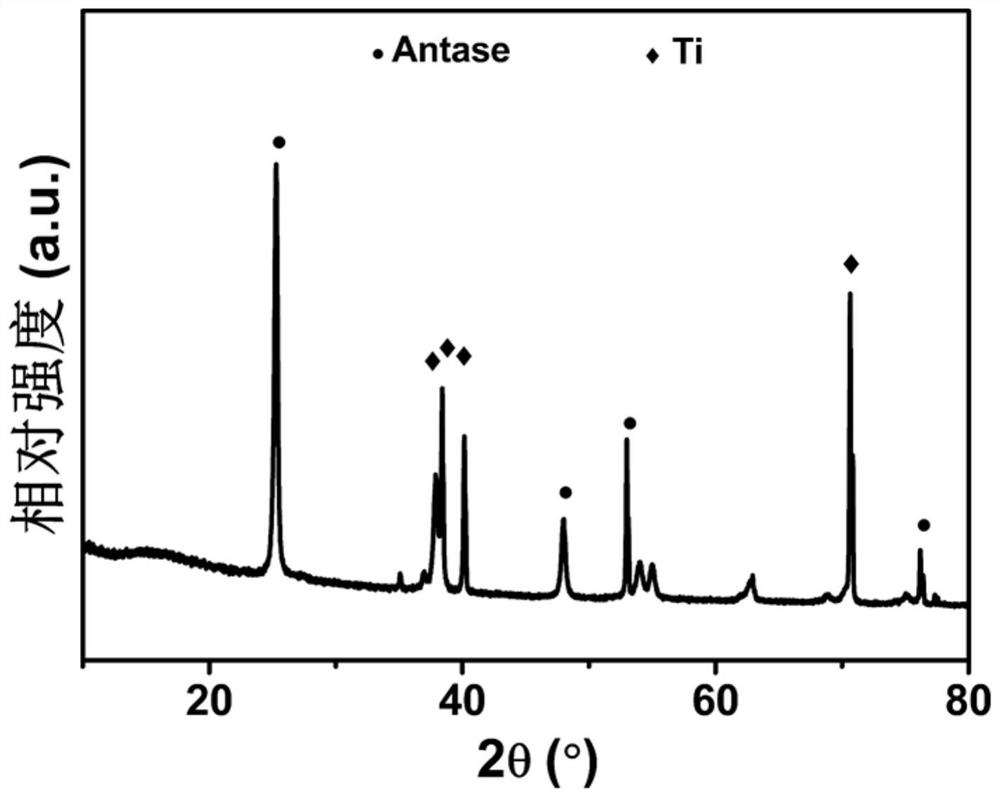
[0042] The Ti sheet was cut to a size of 3cm×3.5cm, immersed in an ethylene glycol solution containing 3.109g / L ammonium fluoride and 11.75% (volume ratio) deionized water under a two-electrode system, and a platinum electrode was used as a counter electrode at 50V Voltage etching for 6 hours. Will get TiO 2 The nanotube precursor was placed in a muffle furnace and calcined at 450 °C for 60 min to obtain anatase TiO 2 nanotube.
[0043] (2) Preparation of electrochemical self-doping TiO 2 nanotube
[0044] The material obtained in step (1) is used as a working electrode, the saturated calomel electrode is used as a reference electrode, the platinum sheet electrode is used as a counter electrode, and the electrolyte is 0.1M Na 2 SO 4 ...
Embodiment 2
[0050] A highly active electrochemical self-doped TiO 2 Preparation of Nanotube-Based Materials and Its Gas-Phase Photoelectrocatalytic Reduction of CO 2 method, including the following steps:
[0051] (1) Preparation of TiO 2 nanotube
[0052] The Ti sheet was cut to a size of 3cm×3.5cm, immersed in an ethylene glycol solution containing 3.109g / L ammonium fluoride and 11.75% (volume ratio) deionized water under a two-electrode system, and a platinum electrode was used as a counter electrode at 60V Voltage etching for 6 hours. Will get TiO 2 The nanotube precursor was placed in a muffle furnace and calcined at 450 °C for 60 min to obtain anatase TiO 2 nanotube.
[0053] (2) Preparation of electrochemical self-doping TiO 2 nanotube
[0054] The material obtained in step (1) is used as the working electrode, the saturated calomel electrode is used as the reference electrode, the platinum sheet electrode is the counter electrode, and the electrolyte is 0.1M K 2 SO 4 sol...
Embodiment 3
[0060] A highly active electrochemical self-doped TiO 2 Preparation of Nanotube-Based Materials and Its Gas-Phase Photoelectrocatalytic Reduction of CO 2 method, including the following steps:
[0061] (1) Preparation of TiO 2 nanotube
[0062] The Ti sheet was cut to a size of 3cm×3.5cm, immersed in an ethylene glycol solution containing 3.109g / L ammonium fluoride and 11.75% (volume ratio) deionized water under a two-electrode system, and a platinum electrode was used as a counter electrode at 60V Voltage etching for 6 hours. Will get TiO 2 The nanotube precursor was placed in a muffle furnace and calcined at 450 °C for 60 min to obtain anatase TiO 2 nanotube.
[0063] (2) Preparation of electrochemical self-doping TiO 2 nanotube
[0064] The material obtained in step (1) is used as the working electrode, the saturated calomel electrode is used as the reference electrode, the platinum sheet electrode is the counter electrode, and the electrolyte is 0.5M K 2 SO 4 sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| power | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com