Method for selectively leaching metal components in waste lithium cobalt oxide battery by using polyethylene glycol-citric acid solvent
A technology of polyethylene glycol and metal components, which is applied in the field of selective leaching of metal components of waste lithium cobaltate batteries with mixed solvents, can solve the problems of inability to separate active materials, and achieve high leaching efficiency, strong selectivity, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
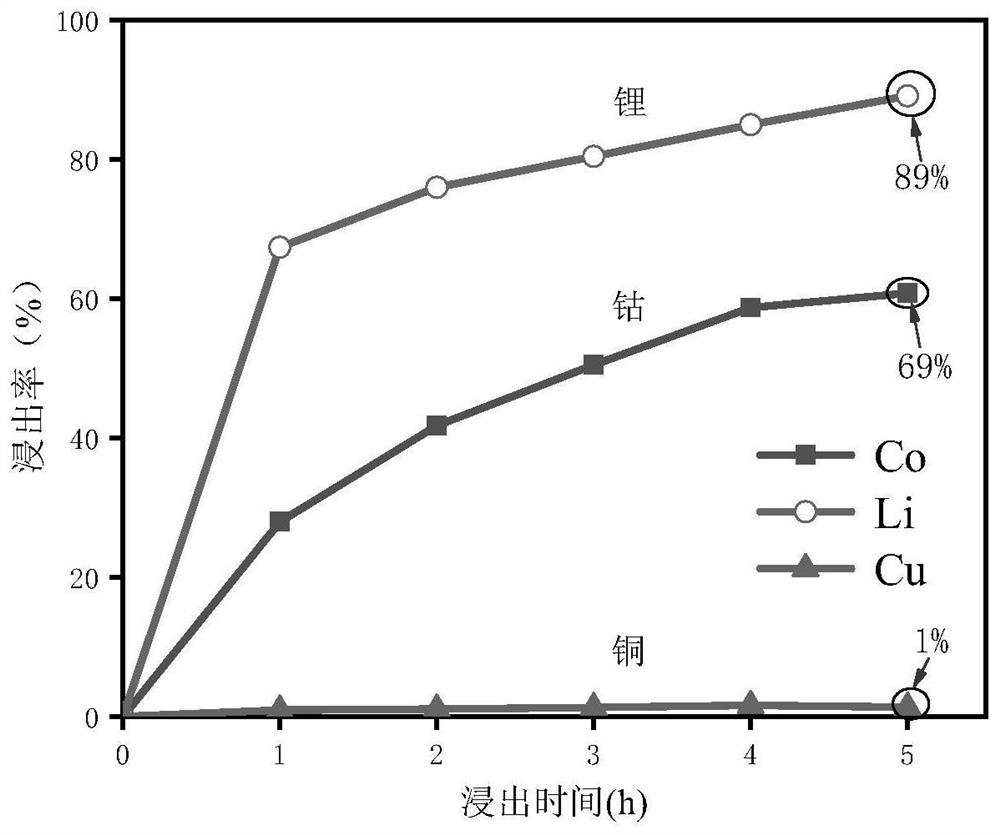
[0038] A kind of mixed solvent that is made up of polyethylene glycol-citric acid is used for selectively leaching metal in lithium cobalt oxide battery, and concrete implementation steps are as follows:
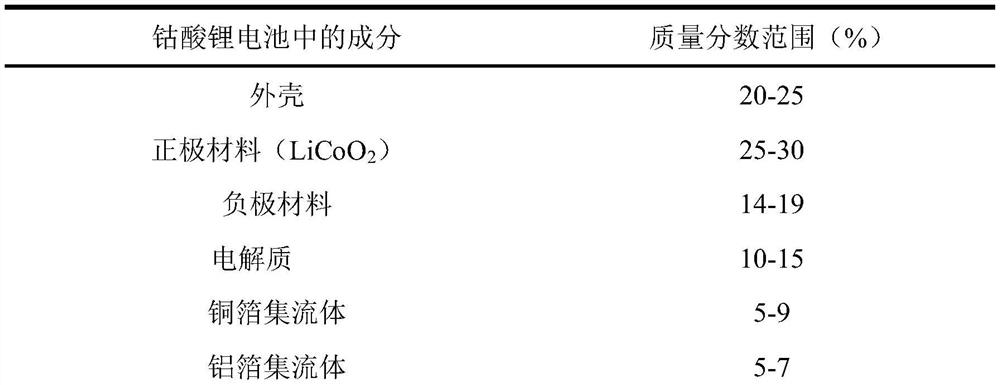
[0039] 1) Weigh lithium cobalt oxide powder and copper powder (200 mesh), and configure materials with a mass ratio of 2;
[0040] 2) After mixing polyethylene glycol 200 and citric acid at a molar ratio of 1:1 at room temperature, stir at 80°C in a collector-type water bath magnetic stirrer until a clear and transparent solution is obtained to prepare a solvent ;
[0041] 3) Add the material obtained in step (1) into the solvent obtained in step (2) according to the mass ratio of 1:1000, stir at 80°C, and leaching for 5h;
[0042] 4) During the leaching period, a small amount of the mixed solution was taken out at intervals of 1 hour, diluted ten times with water, and the insoluble matter was filtered to obtain a liquid sample.
[0043] 5) Using atomic absorption spectromet...
Embodiment 2
[0045] 1) Weigh lithium cobalt oxide powder and copper powder (200 mesh), and configure materials with a mass ratio of 2:1;
[0046] 2) After mixing polyethylene glycol 200 and citric acid at a molar ratio of 2:1 at room temperature, stir in a collector-type water bath magnetic stirrer at 60°C until a clear and transparent solution is obtained to prepare a solvent ;
[0047] 3) Add the material obtained in step (1) into the solvent obtained in step (2) according to the mass ratio of 1:500, stir at 80°C, and leaching for 5 hours;
[0048] 4) During the leaching period, a small amount of mixed solution was taken out at intervals of 1 hour, added to dilute ten times, and the insoluble matter was filtered out to obtain a liquid sample.
[0049] 5) Using atomic absorption spectrometry to analyze the metal content of the liquid extracted at different reaction times. The results showed that when the leaching time was 3 hours, the lithium metal content in the reaction gained liquid ...
Embodiment 3
[0051] 1) Weigh lithium cobalt oxide powder and copper powder (200 mesh), and configure materials with a mass ratio of 2:1;
[0052] 2) After mixing polyethylene glycol 200 and citric acid at a molar ratio of 1:1 at room temperature, stir at 80°C in a collector-type water bath magnetic stirrer until a clear and transparent solution is obtained to prepare a solvent ;
[0053] 3) Add the material obtained in step (1) into the solvent obtained in step (2) according to the mass ratio of 1:100, stir at 100°C, and leaching for 8 hours;
[0054] 4) During the leaching period, a small amount of the mixed solution was taken out at intervals of 1 hour, diluted ten times with water, and then the insoluble matter was filtered to obtain a liquid sample.
[0055] 5) After the solution is prepared, the metal content of the liquid extracted at different reaction times is analyzed by atomic absorption spectrometry. Adopt standard curve method to measure, be 2 hours in leaching time, lithium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com