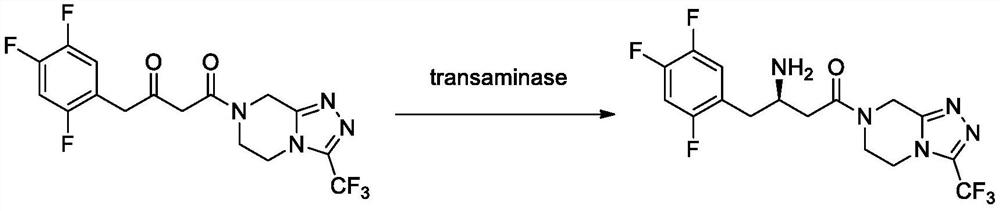
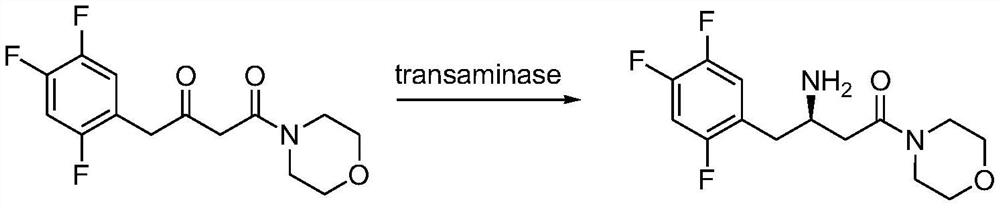
Transaminase mutant, immobilized transaminase and application of immobilized transaminase to preparation of sitagliptin
A technology for immobilizing transaminase and sitagliptin, applied in the preparation of sitagliptin or its intermediates, in the field of preparation of sitagliptin, can solve the problem of high conversion rate, non-recyclable use and low activity of transaminase , Enzyme activity is not high, etc., to achieve the effect of increased reusability, simple operation, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Embodiment 1 A kind of preparation of transaminase mutant enzyme liquid
[0080] The genes shown in the nucleotide sequences of SEQ ID NO.2, SEQ ID NO.4, SEQ ID NO.6, and SEQ ID NO.8 were synthesized, and the gene preparation company was Suzhou Jinweizhi Biotechnology Co., Ltd. (Suzhou Industrial Park Building C3, Bio-Nano Technology Park, No. 218 Xinghu Street). The above-mentioned genes respectively code for SEQ ID NO.1 in the sequence list (ie, SEQ ID NO:110 in US8293507), SEQ ID NO.3, SEQ ID NO.5 (ie, SEQ ID NO:130 in US9617573), SEQ ID NO .7 Transaminases indicated.
[0081] table 3
[0082]
[0083]
[0084] Then synthesize gene enzyme-linked pET28a, restriction site NdeI&HindIII, and enzyme-linked vector to transform host Escherichia coli BL21 competent cells. Inoculate the constructed strain into TB culture based on 37°C, 200rpm shaker, induce overnight with IPTG concentration of 0.1mM, collect the strains, and obtain engineering strains containing tran...
Embodiment 2
[0102] Embodiment 2 Preparation of immobilized transaminase mutant
[0103] Take 12g of transaminase mutant sludge, add 120mL of 100mM phosphate buffer, mix evenly under high pressure and homogeneously break the cells, centrifuge to collect the supernatant enzyme solution, add 22gK 2 HPO 4 ·3H 2 O, 2.2gKH 2 PO 4 and 0.024g of PLP, stir to dissolve, add 10g of resin (see Table 5 for details of resin type, all purchased from Suzhou Huitong Chromatography Separation and Purification Co., Ltd.) Immobilized enzyme was obtained by suction filtration. The enzyme activity detection of the immobilized enzyme was carried out with morpholinodione and sitadione as substrates respectively, and the enzyme activity detection method was as follows:
[0104] Weigh 1.2g of morpholinodione or sitadione substrate into a 50mL Erlenmeyer flask, add 19mL of isopropanol, 0.6mL of 16mg / mL PLP solution and 0.4mL of isopropylamine, shake on a shaker at 45°C until it is completely dissolved, and coo...
Embodiment 3
[0109] Immobilized enzyme (resin: EC HFA) for the catalysis of morpholinodione
[0110] In the conical flask, add 25mL isopropanol, 2.5g morpholino, 2mL water, 32mgPLP, 1.135mL isopropylamine, 10g immobilized enzyme prepared in Example 2, and react on a shaking table at 45°C and 200rpm. After 24 hours of reaction, samples were taken to detect the conversion rate. Filter the reaction liquid to obtain immobilized enzyme, continue to add 25mL of isopropanol, 2.5g of morpholino, 2mL of water, 32mg of PLP, 1.135mL of isopropylamine, 45 ℃, 200rpm shaker reaction, react for 24 hours and take samples to detect the conversion rate. The immobilized enzyme was repeated according to the above method. The results are shown in Table 6, which shows that the conversion rate of Enz.1-M122Q-P233T and Enz.2-M122F remained above 85% and the ee value was >99.9%. Enzase is stable; Enz.1 and Enz.2 (the effect is not good after applying mechanically for 3 times) have low conversion rate and are n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com