Interworking ligand, hydroformylation catalyst and preparation method of dihydric alcohol
A hydroformylation catalyst and hydroformyl technology are applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., and can solve the problems of low reaction yield and the like, Achieve high reactivity, good linear selectivity, and reduce equipment investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Preparation of catalyst
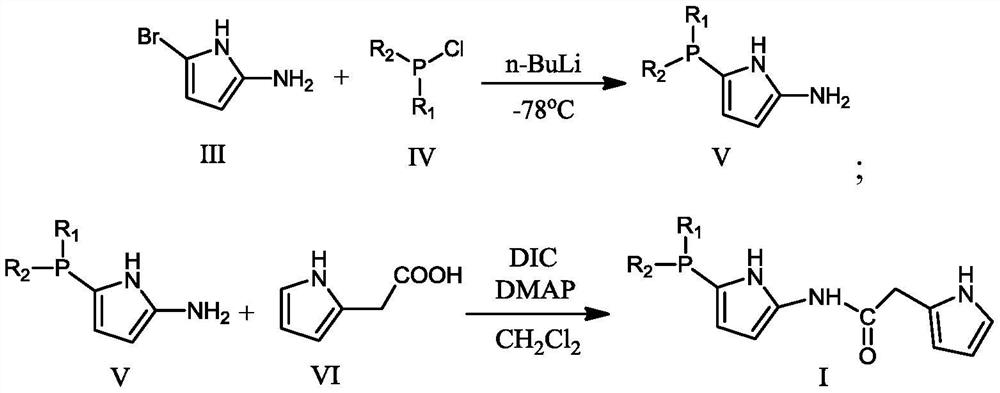
[0053] Ligand I: CH to 5-bromo-2-aminopyrrole (67.94g, 0.422mol) at -78°C under argon atmosphere 2 Cl 2 (1.5 L) n-BuLi (29.75 g, 0.4645 mol) was slowly added to the solution. The mixture was stirred for 30 min, then Ph 2 PCl (109.63g, 0.4965mol), continue to react at -78°C for 1.5 hours, then warm up to room temperature, and stir at this temperature for another 2 hours to obtain intermediate V (106.5g, 0.4mol); then add pyrrole-2 - Acetic acid (75.08g, 0.6mol), DMAP (48.9g, 0.4mol), DIC (75.72, 0.6mol), react at room temperature for 4 hours to obtain the product (145.62g, 0.39mol).
[0054]
[0055] Elemental analysis: C: 70.79; H: 5.38; N: 11.23; O: 4.30; P: 8.30.
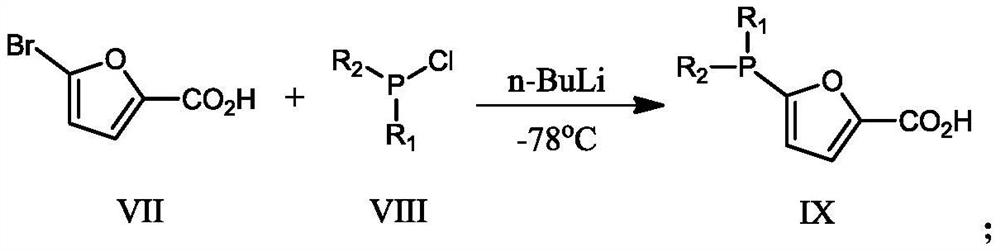
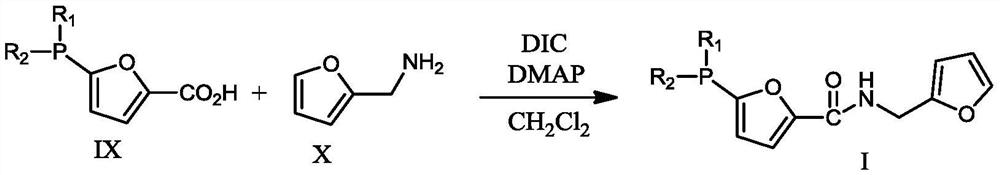
[0056] Ligand II: CH to 5-bromofuran-2-carboxylic acid (80.59g, 0.422mol) at -78°C under argon atmosphere 2 Cl 2 (1.5 L) n-BuLi (29.75 g, 0.4645 mol) was slowly added to the solution. The mixture was stirred for 30 min, then Ph 2 PCl (109.54g, 0.4965mol), continued t...
Embodiment 2
[0062] (1) Preparation of catalyst
[0063] Ligand I: The preparation steps are the same as in Example 1, except that the Ph in Example 1 2 PCl (109.54g, 0.4965mol) was changed to C 8 h 8 S 2 PCl (115.53 g, 0.4965 mol). The product (180.73 g, 0.4 mol) was finally obtained.
[0064]
[0065] Elemental analysis: C: 58.59; N: 10.28; H: 3.92; S: 15.62; O: 3.92;
[0066] Ligand II: The preparation steps are the same as in Example 1, except that the Ph in Example 1 2 PCl (109.54g, 0.4965mol) was changed to C 8 h 8 S 2 PCl (115.53 g, 0.4965 mol). The product (151.09 g, 0.39 mol) was finally obtained.
[0067]
[0068] Elemental analysis: C: 55.79; N: 3.65; H: 3.62; O: 12.41; P: 8.02;
[0069] (2) propene hydroformylation to prepare n-butanol
[0070] Add 300 g of benzene, 0.2 g of rhodium octanoate, 2 g of ligand I, and 2.42 g of ligand II into an autoclave with a stirrer and a thermometer. 2 : CO = 1:1 synthesis gas replacement 3 times, then add propylene to 1.5MP...
Embodiment 3
[0072] (1) Preparation of catalyst
[0073] Ligand I: The preparation steps are the same as in Example 1, except that the Ph in Example 1 2 PCl (109.54g, 0.4965mol) was changed to C 8 h 8 N 2 PCl (110.22, 0.4965 mol). The product 137.02 g (0.39 mol) was finally obtained.
[0074]
[0075] Elemental analysis: C: 61.50; N: 19.92; H: 5.14; O: 4.55; P: 8.89.
[0076] Ligand II: The preparation steps are the same as in Example 1, except that the Ph in Example 1 2 PCl (109.54g, 0.4965mol) was changed to C 8 h 6 S 2 PCl (115.53 g, 0.4965 mol). The product (151.09 g, 0.39 mol) was finally obtained.
[0077]
[0078] Elemental analysis: C: 55.79; N: 3.65; H: 3.62; O: 12.41; P: 8.02;
[0079] (2) Hydroformylation of 1,3-butadiene to prepare 1,6-hexanediol
[0080] Add 300 g of benzene, 0.2 g of cobalt acetate, 2 g of ligand I, and 3.3 g of ligand II into an autoclave with a stirrer and a thermometer, and use H in the autoclave to 2 :CO=1:1 synthesis gas replacement 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com