Iron-nickel alloy resource recycling method and application
A nickel-iron alloy and resource-based technology, applied in chemical instruments and methods, inorganic chemistry, phosphorus compounds, etc., can solve problems such as unfavorable promotion, increased cost, and increased extraction process, and achieve short process flow, low equipment requirements, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
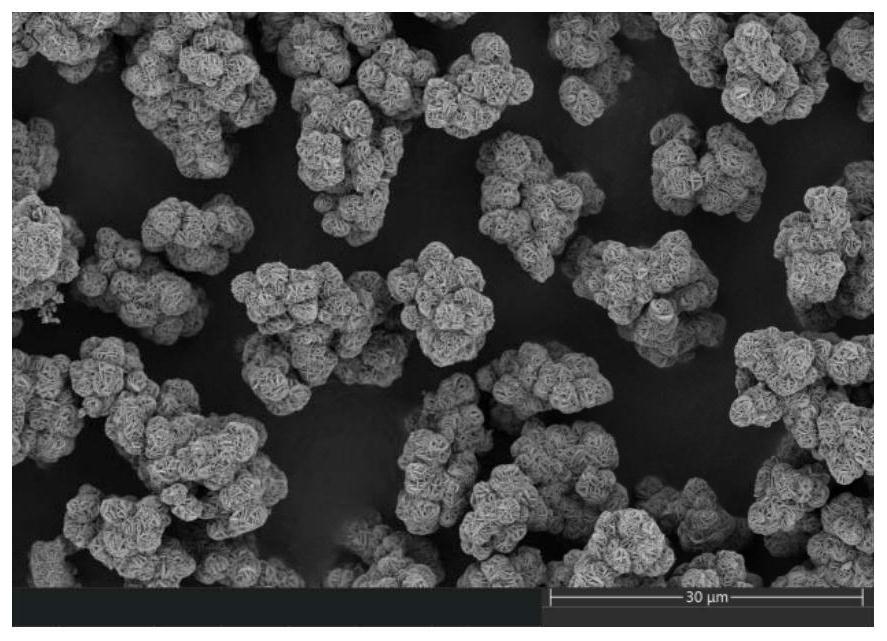
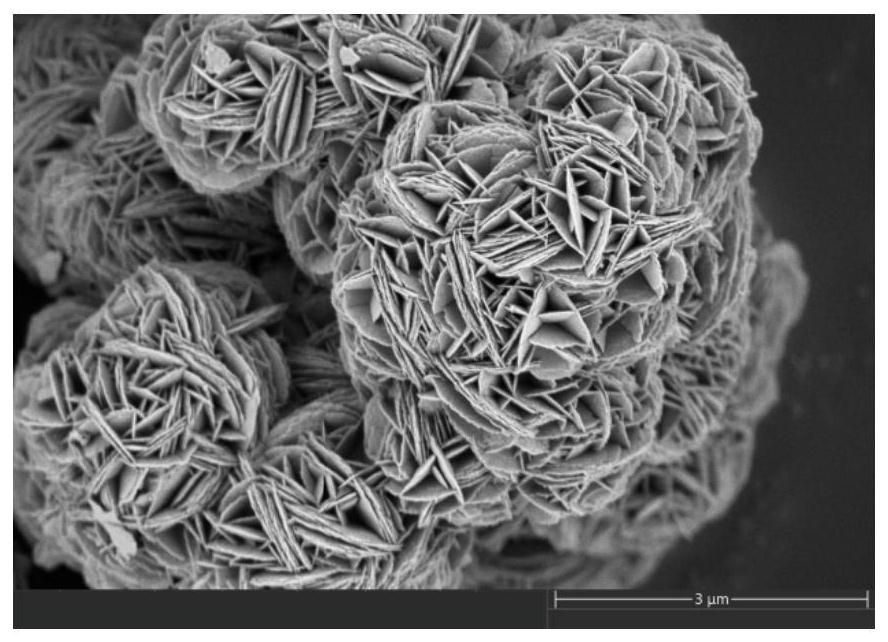
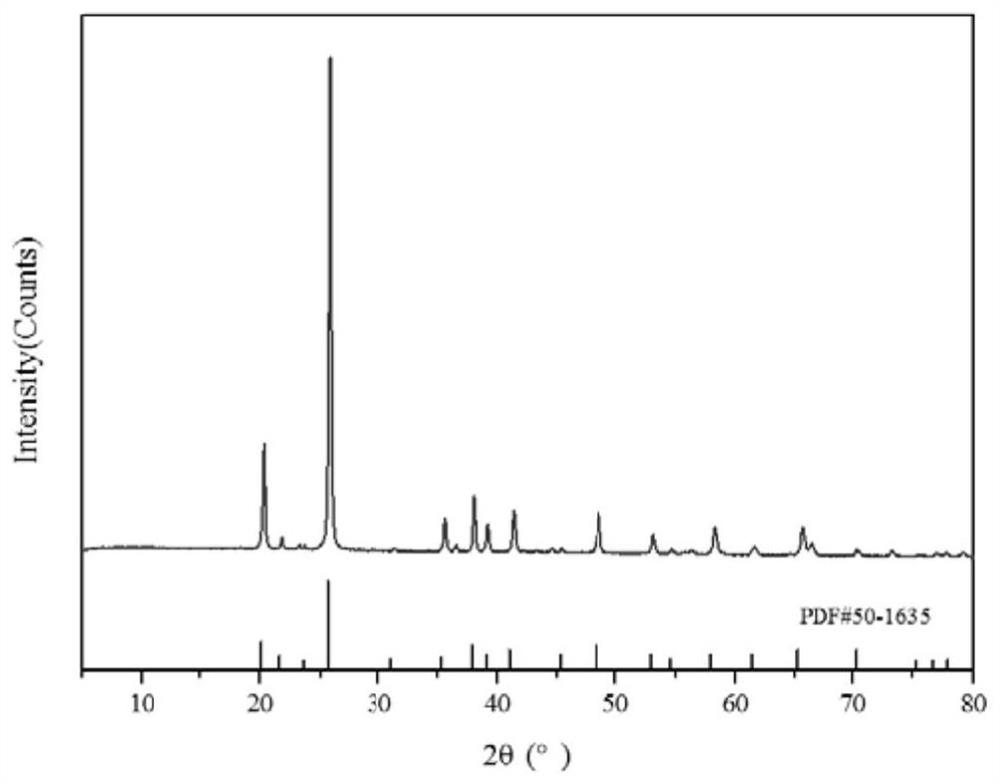
Image
Examples
Embodiment 1
[0058] The method for resource recycling of nickel-iron alloy of the present embodiment comprises the following steps:
[0059] (1) A nickel-iron alloy block of 1mm to 60mm (iron: 63.28%, nickel: 35.51%, Co: 0.34%, Mn: 0.09%, Si: 0.27%, Cr: 0.05%, Ca: 0.006%, Mg: 0.004 %, Cu: 0.03%, S: 0.21%, P: 0.03%) are ball milled, pulverized, and sieved to obtain ferronickel powder;
[0060] (2) Mix 100 g of ferronickel powder with 1 L of sulfuric acid with a concentration of 1.5 mol / L, heat to 90° C., react for 4 hours, and filter to obtain ferronickel-containing leaching solution and leaching residue;
[0061] (3) Add 0.9 times the theoretical amount of sodium phosphate to the leaching solution obtained in step (2), stir for 1 hour, add 1.3 times the theoretical amount of hydrogen peroxide, stir for 2 hours, then add 50 g of anhydrous iron phosphate, heat to 80 ° C, and react 6h, filtered to obtain ferric phosphate and precipitated liquid;
[0062] (4) pulping and washing the iron pho...
Embodiment 2
[0068] The method for resource recycling of nickel-iron alloy of the present embodiment comprises the following steps:
[0069] (1) 200g nickel-iron alloy block (iron: 63.28%, nickel: 35.51%, Co: 0.34%, Mn: 0.09%, Si: 0.27%, Cr: 0.05%, Ca: 0.006%, Mg: 0.004%, Cu : 0.03%, S: 0.21%, P: 0.03%) mixed with 1L of sulfuric acid with a concentration of 1.5mol / L, heated to 80°C, reacted for 12 hours and filtered to obtain the leach solution containing ferronickel and unreacted ferronickel alloy block ;
[0070] (2) adding 1.0 times of theoretical amount of phosphoric acid to the leaching solution obtained in step (2), after stirring for 0.5h, feed oxygen, and the reaction time is 5h;
[0071] (3) Add 100 g of ferric phosphate dihydrate to the ferric phosphorus solution obtained in step (3), heat to 90° C., react for 4 hours, and filter to obtain ferric phosphate and precipitated liquid;
[0072] (4) pulping and washing the ferric phosphate obtained in step (4) for 2 hours, drying fer...
Embodiment 3
[0075] The method for resource recycling of nickel-iron alloy of the present embodiment comprises the following steps:
[0076] (1) The nickel-iron alloy block (iron: 83.12%, nickel: 15.45%, Co: 0.51%, Mn: 0.05%, Si: 0.36%, Cr: 0.09%, Ca: 0.012%, Mg: 0.008%, Cu: 0.05%, S: 0.22%, P: 0.01%) were ball milled and sieved to obtain ferronickel powder;
[0077] (2) 200g ferronickel powder is mixed with 0.8L concentration of nitric acid of 2.5mol / L, heated to 85°C, reacted for 5h and then filtered to obtain ferronickel-containing leaching solution and leaching slag;
[0078] (3) Add 0.93 times the theoretical amount of ammonium phosphate to the leaching solution obtained in step (2), and mix and stir for 2 hours;
[0079] (4) Add 20 g of alumina and iron phosphate dihydrate to the iron phosphorus solution obtained in step (3), heat to 70° C., react for 12 hours, and filter to obtain iron phosphate and precipitated liquid;
[0080] (5) pulping and washing the ferric phosphate obtaine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com