Preparation method of polymerized 3-hydroxybutyric acid
A technology of hydroxybutyric acid and polymer, which is applied in the field of polymerizing 3-hydroxybutyric acid preparation, can solve the problems of harsh conditions, serious pollution, unfavorable industrial production, etc., and achieve the effect of increased yield and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
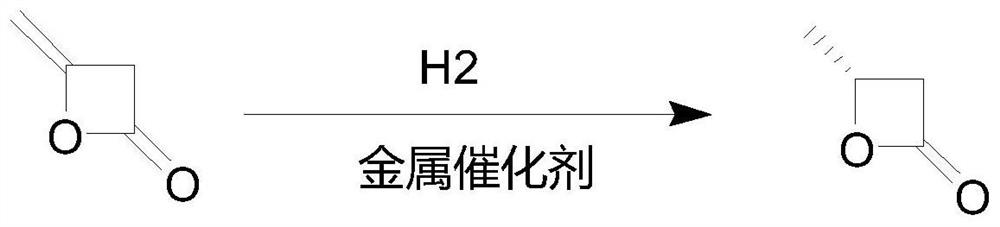
[0018] In the hydrogenation reaction pot, add 84.07 grams of diketene, 160 ml of dichloroethane, replace with nitrogen 3 times, then raise the temperature to 50°C while feeding hydrogen, keep the pressure at 2.0MPa, and react under the conditions until the hydrogen is no longer consumed , terminated the reaction and lowered the temperature, and evaporated ethanol to obtain 81.8 grams of β-butyrolactone.
[0019] Add 81.8 grams of β-butyrolactone, 475 grams of water, 0.4 grams of biological enzyme catalyst, and 0.4 grams of magnesium octanoate in the flask, then raise the temperature to 120°C for reaction, take samples to detect the products in the reaction system, stop the reaction after passing the test, cool down and filter to obtain 77.8 grams of 3-hydroxybutyric acid was polymerized, and 472 grams of filtrate obtained by filtration was also applied to the next batch of polymerization in step (2). The total yield of the two steps was 92.5%.
Embodiment 2
[0021] In the hydrogenation reaction pot, add 84.07 grams of diketene and 250 milliliters of dichloroethane, use nitrogen to replace 3 times, then cool down to -10°C while feeding hydrogen, keep the pressure at 0.1MPa, and react under the conditions until it is no longer consumed Hydrogen, stop the reaction and lower the temperature, evaporate ethanol to obtain 80 grams of β-butyrolactone.
[0022] Add 80 grams of β-butyrolactone in the flask, add 472 grams of filtrate water in Example 1, add 5 grams of fresh water, add 0.5 grams of biological enzyme catalyst, 0.3 grams of magnesium octanoate, then heat up to 50 ° C for reaction, and take samples for detection The product in the reaction system was qualified and the reaction was terminated, and the temperature was lowered and filtered to obtain 78.2 grams of polymerized 3-hydroxybutyric acid, and the total yield of the two steps was 93.01%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com