Amorocoelophoma sp. and application thereof in preparation of spirolactone derivative
A technology of plant endophytic fungi and derivatives, which is applied to the application field of plant endophytic fungi and the preparation of spirolactone derivatives, which can solve the problem that Amorocoelophoma has no secondary metabolites and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
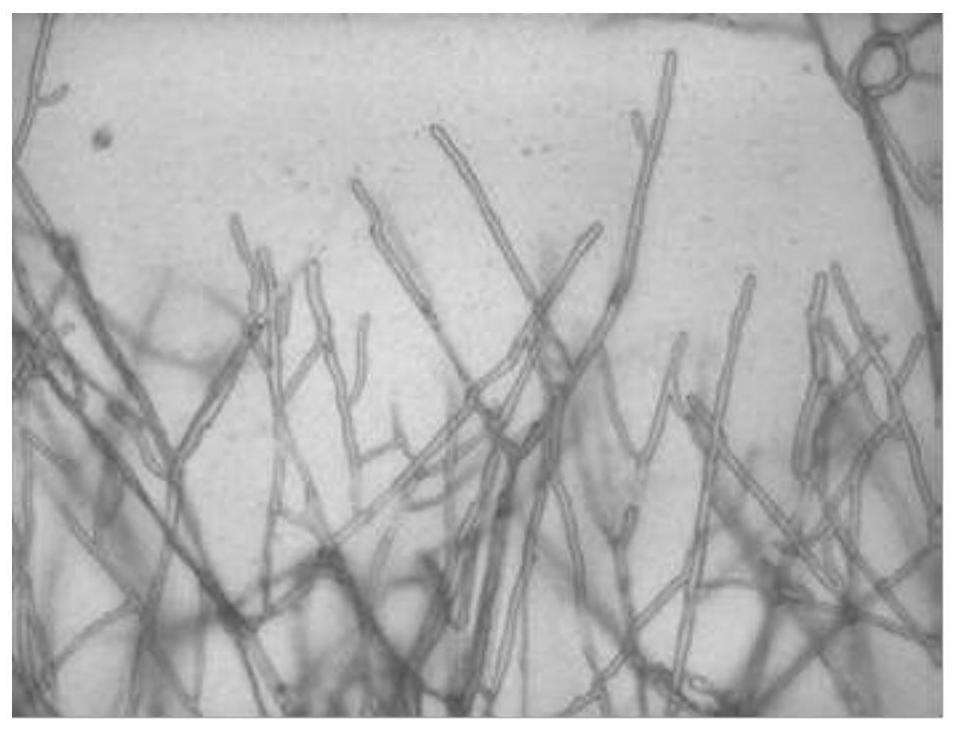
[0032] (1) Rinse the freshly collected, non-conditioned Yunnan Stone Skin with tap water, remove the soil and dust of the surface. The dried plant material was placed in an ultra-clean workbench through UV-sterilized, and 75% alcohol and 2% sodium hypochlorite were selected as surface disinfectants. The plant materials were first soaked with 75% alcohol for 30-60S, rinsed 2 times in sterile water; then treated with 2% sodium hypochlorite, 1.5-3 min, sterile water rinsing 3 times. Sterile filter paper suction moisture.
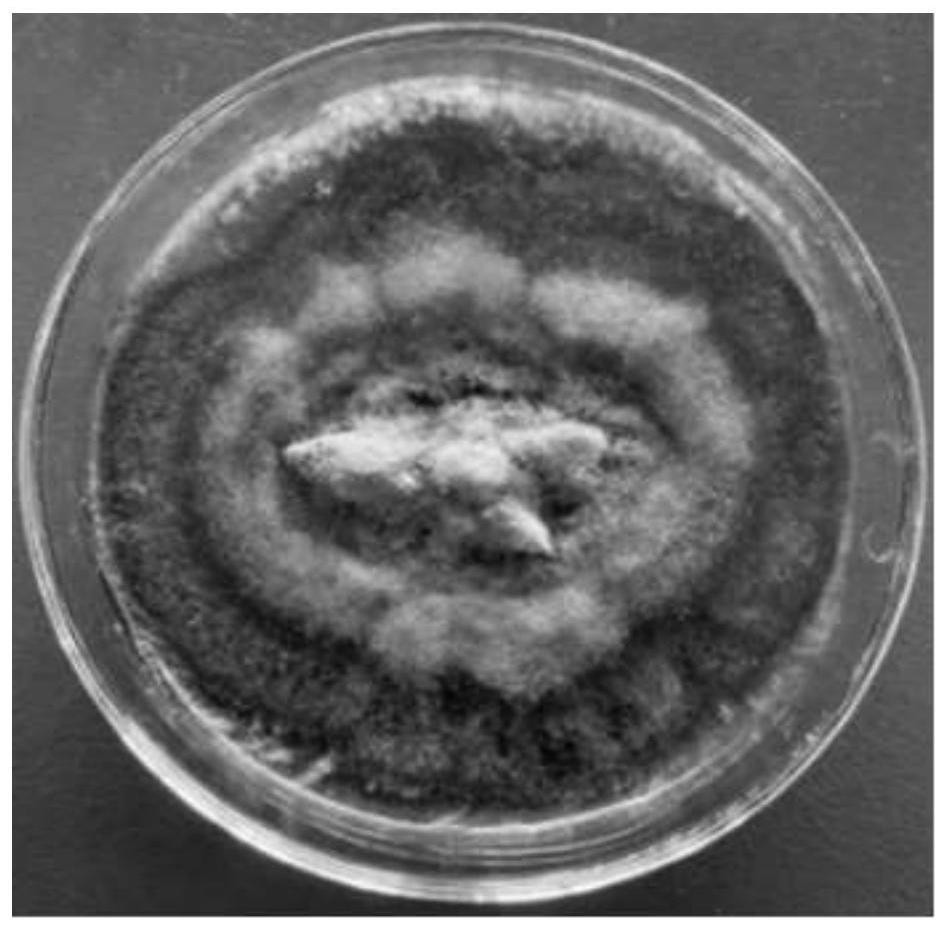
[0033] (2) The Yunnan Stone Timberry, which is disinfected with step (1), has a small piece of a small piece of about 1 cm, and the small piece of plant tissue is inoculated on the PDA medium, and the temperature is cultured at 28 ± 2 ° C. The PDA medium includes 200 g of peel potatoes, 20 g of glucose, grease 15g, distilled water 1000ml, pH nature.
[0034] (3) Observe the growth of the surface of the plant tissue block, and use a sterile bamboo stick to pick up t...
Embodiment 2
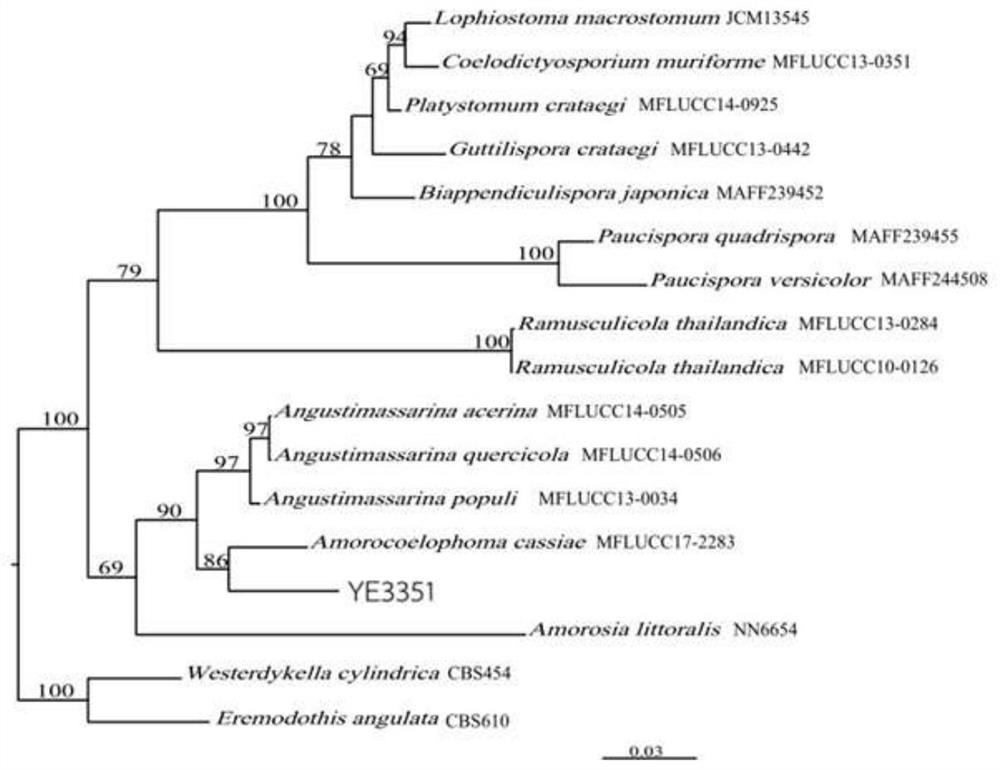
[0039] Amorocoelophoma sp.YE3351 Method for fermenting helium lysate derivatives, including the following steps:
[0040](1) PDB medium: weighing 200g, cut into small pieces, add 1000 mL of distilled water to boil, gauze filtrate, gauze, pH 20g, pH, 121 ° C Sterilization for 30 min, produce PDB medium Near, where the concentration of potatoes in the PDB medium is 0.2 g / ml, and the concentration of glucose is 0.02 g / ml.
[0041] (2) AmorocoElophoma sp.YE3351 strain is incorporated into a PDB medium, and 4D is cultured at 28 ± 2 ° C, 200R / min shaker, and the seed fluid is obtained, and then the resulting seed liquid is added to 10% inoculation amount. In the medium, 7d was cultured in 28 ± 2 ° C, 200R / min shaker, and the fermentation product was obtained.
[0042] (3) Filting the fermentation product obtained by step (2) is filtered, resulting in fermentation liquid and mycelial body, the fermentation broth extraction, mycerer is extracted with methanol, combined with EtOAc,...
Embodiment 3
[0051] Anti-viral activity assay for Barnocycloalkyl derivative Massarilactone D and MassariLACTONE E:
[0052] (1) Determination of Cell Disease (CPE):
[0053] CPE analysis is used to evaluate the anti-H1N1 influenza virus activity of the helicy cyclic derivative Massarilactone D and Massarilactone E. Collect MDCK cells and count, 20ml 10 by 2% DMEM 5 / ml cells, MDCK cells in 2 × 10 4 The density of a cell / well is inoculated into a 96-well plate and incubated at 37 ° C, and in 5% CO at 37 ° C. 2 Place 24h in. Two-fold continuous diluted spiral lacene derivative Massarilactone D and Massarilactone E and Hydrotective virus (100TCID 50 Mix, incubated at 37 ° C for 30 min, then transferred to a cell in a 96-well plate, and then incubated for 30 min. After incubation, MDCK cells were washed twice with PBS to remove uncomfortable viruses, and then added 1 μg / ml TPCK-trypsin and 0.2% BSA DMEM. After incubation 48 h, an antiviral effect was observed under a microscope. The data was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com