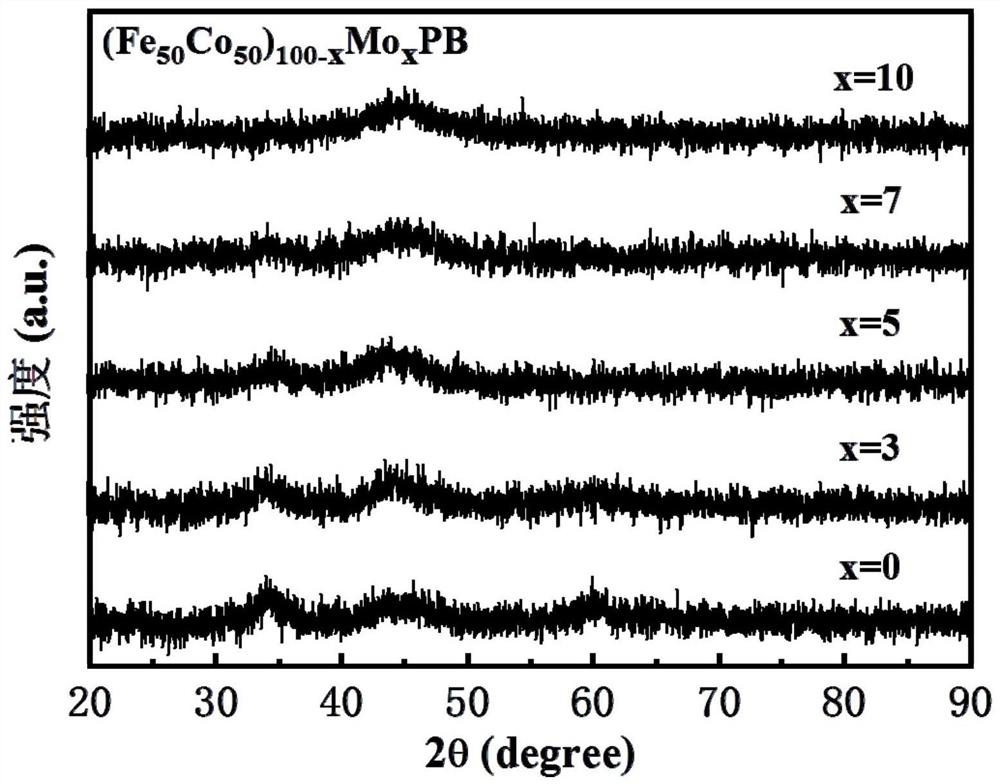
FeCoMoPB amorphous nanoparticle oxygen evolution catalyst and preparation method thereof
A nanoparticle and catalyst technology, applied in electrodes, electrolysis processes, electrolysis components, etc., can solve the problems of poor stability, low oxygen evolution catalytic performance, etc., and achieve improved structural stability and durability, simple preparation process, and easy operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation (Fe 50 co 50 ) 97 Mo 3 PB amorphous nanoparticles, the preparation process is as follows:
[0034] Step 1, weigh 0.2893g of ferrous chloride tetrahydrate, 0.3461g of cobalt chloride hexahydrate, 0.0218g of sodium molybdate dihydrate, and 0.9539g of sodium hypophosphite monohydrate, mix them evenly, and dissolve them completely with 50mL of deionized water, stir Uniformly, the reaction precursor solution is obtained;
[0035] Step 2, the above-mentioned precursor solution is transferred to a three-necked flask, during which high-purity nitrogen is introduced to remove oxygen in the solution;
[0036] Step 3, weigh 0.3405g of sodium borohydride, dissolve it in 15mL of deionized water, and configure it into a fresh solution of 0.6mol / L sodium borohydride;
[0037] Step 4, under strong mechanical stirring and high-purity nitrogen protection, add sodium borohydride solution dropwise to the precursor solution in the three-necked flask, and the dropping rate i...
Embodiment 2
[0045] Preparation with reference to Example 1 (Fe 50 co 50 ) 95 Mo 5 PB amorphous nanoparticles, the difference is that in step 1, 0.2833g of ferrous chloride tetrahydrate, 0.3390g of cobalt chloride hexahydrate, 0.0363g of sodium molybdate dihydrate, and 0.9539g of sodium hypophosphite monohydrate were weighed and mixed uniformly.
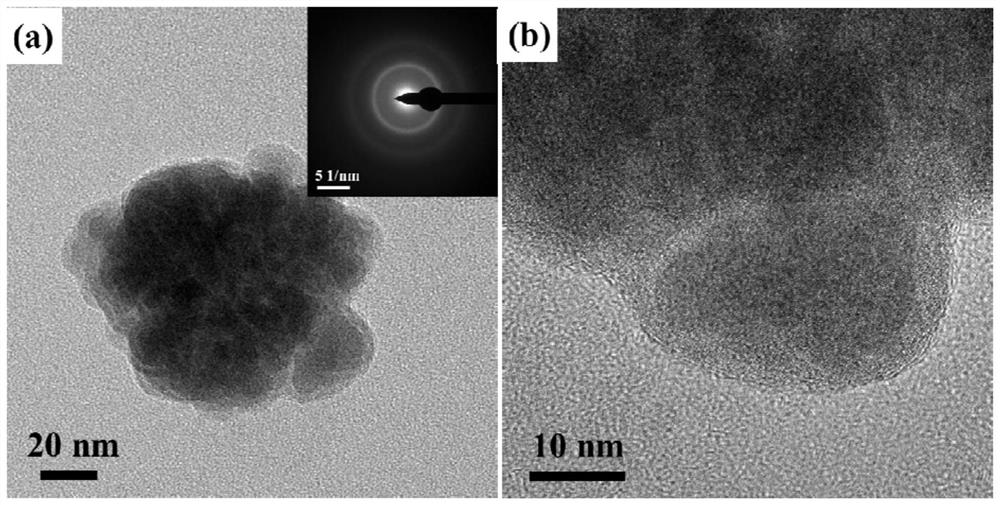
[0046] The prepared (Fe 50 co 50 ) 97 Mo 3 The morphology and structure of PB amorphous nanoparticles are as follows: figure 1 . Depend on figure 1 (a) In the transmission electron microscope (TEM) image, it can be seen that there is a secondary structure inside the nano-scale particles, and the small particles aggregate to form larger particles; no obvious diffraction rings or diffraction rings can be seen in the selected area electron diffraction pattern (SAED) Diffraction spots, proving that it is a completely amorphous structure. figure 1 The high-resolution TEM image of (b) shows that the prepared nanoparticles have an obvious core...
Embodiment 3
[0049] Preparation with reference to Example 1 (Fe 50 co 50 ) 93 Mo 7 PB amorphous nanoparticles, the difference is that in step 1, 0.2773g of ferrous chloride tetrahydrate, 0.3319g of cobalt chloride hexahydrate, 0.0508g of sodium molybdate dihydrate, and 0.9539g of sodium hypophosphite monohydrate were weighed and mixed uniformly.
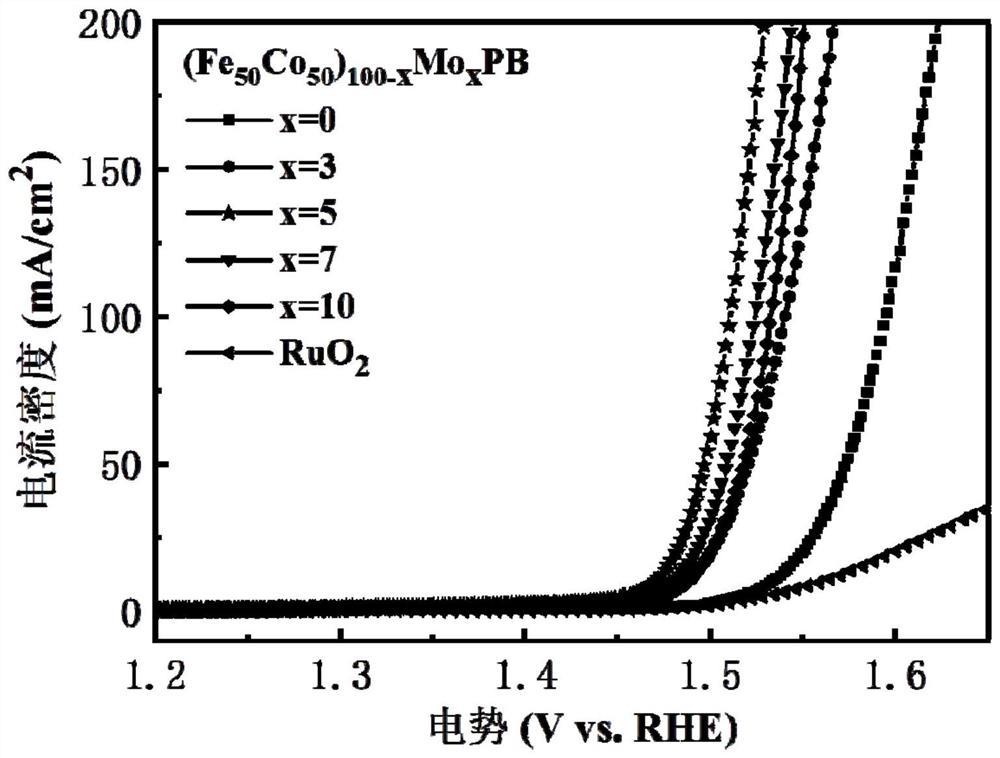
[0050] With reference to the test method of embodiment 1 the (Fe 50 co 50 ) 93 Mo 7 PB amorphous nanoparticles were used as oxygen evolution catalysts for electrolysis of water, and the oxygen evolution catalytic performance was tested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com