Copper-based compound/copper nanoelectrode with interface synergistic effect and preparation and application of copper-based compound/copper nanoelectrode
A synergistic effect, compound technology, applied in electrodes, nanotechnology, electrolysis components, etc., can solve problems such as poor stability, low product selectivity, and difficulty in inhibiting side reactions, and achieve good selectivity, inhibition of hydrogen evolution reaction, and good hydrogen evolution reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] A cuprous oxide / copper nanoelectrode material with interfacial synergistic effect is prepared by the following steps:
[0081] A, prepare reaction solution, prepare the sodium citrate dihydrate of 1.7mol / L, the copper sulfate pentahydrate solution of 2.0mol / L, the sodium hydroxide solution of 5.5mol / L, the ascorbic acid solution of 20mol / L;
[0082] B. Under the stirring process, take 2ml of 1.7mol / L sodium citrate solution in a 500ml beaker (400ml of pure water is housed in the beaker), stir for 20min; add 2ml of 2.0mol / L sulfuric acid pentahydrate to the above solution Copper solution, stir for 5 minutes; add 2ml of 5.5mol / L sodium hydroxide solution to the above mixed solution, stir for 5 minutes; finally add 2ml of 20mol / L ascorbic acid solution into the mixed solution, stir for 30min, centrifuge, wash and vacuum dry , to obtain orange-yellow cuprous oxide powder;
[0083] C. Weigh 3 mg of the cuprous oxide powder obtained in step B and evenly disperse it into 1 ml...
Embodiment 2
[0105] Prepare cuprous oxide / copper nanometer electrode material according to the method described in embodiment 1, and the difference of embodiment 1 is only: square wave potential treatment time is respectively 15 minutes, 30 minutes, 45 minutes and 60 minutes. Finally, four different interface electrode materials were obtained.
[0106] To characterize the 4 interface electrode materials obtained above, see Figure 7a~7d .
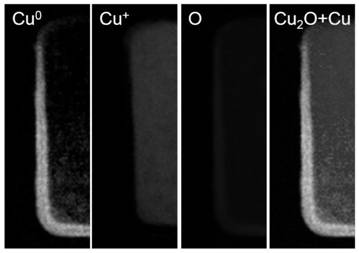
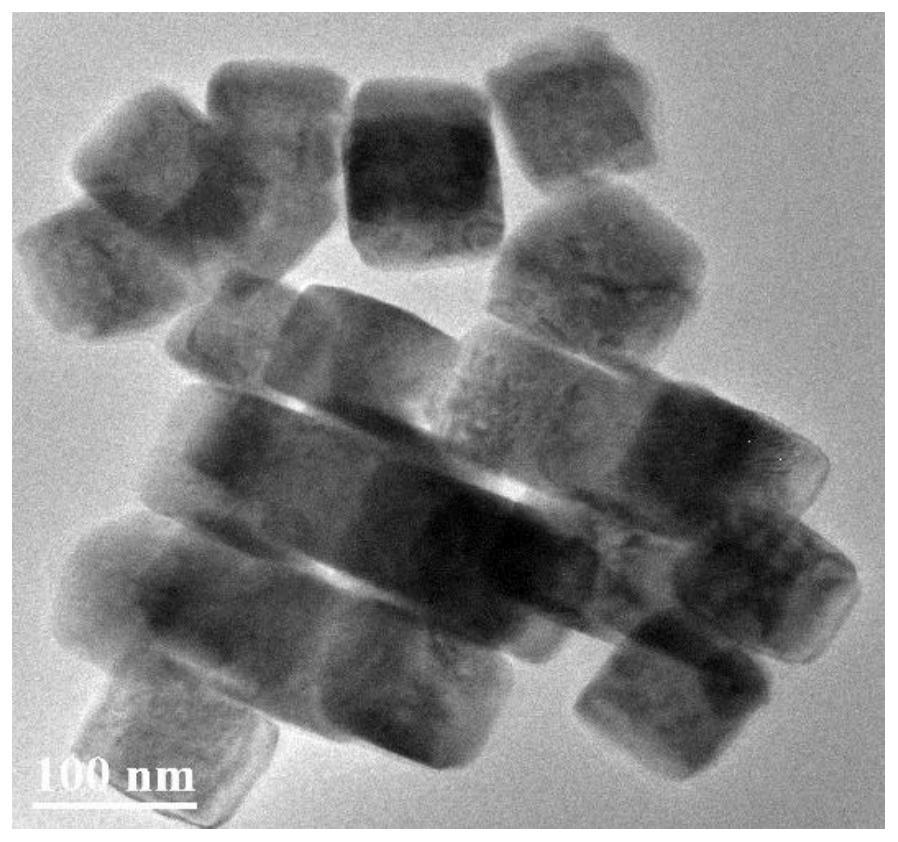
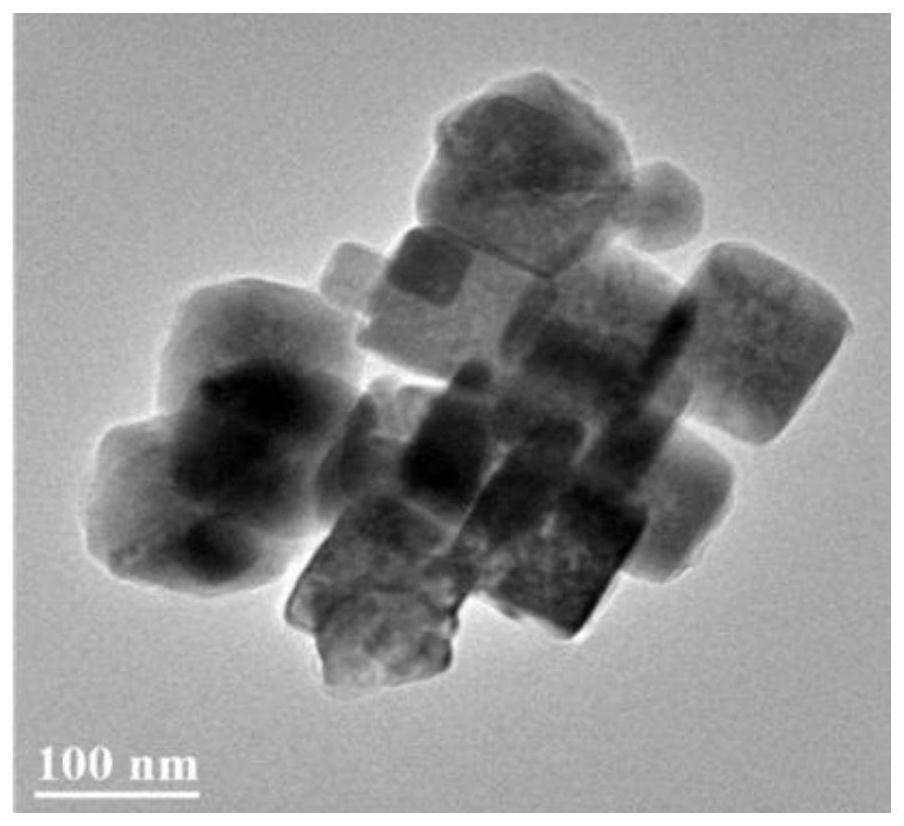
[0107] Figure 7a~7d It is the scanning electron micrograph of the cuprous oxide / copper nanomaterial obtained after the different square wave treatment times of embodiment 2. Wherein a picture is 15 minutes; b picture is 30 minutes; c picture is 45 minutes; d picture is 60 minutes, and the particle diameter of cuprous oxide / copper nanoparticles is about 30~150nm.
[0108] Figure 7a~7d It can be seen that as the processing time of the square wave prolongs, the morphology of the nanomaterials changes continuously. At 15 minutes, it is still a cube si...
Embodiment 3
[0114] Copper nitride / copper nanoelectrode materials with interfacial synergy were prepared by the following methods:
[0115] A. Weigh 100mg of copper acetate and 0.2g of urea and put them in two porcelain boats respectively, keep calcination at 800°C for 3 hours in an argon atmosphere, and place the urea above the air flow at a flow rate of 3L / h. The ammonia gas generated by the decomposition reacts with copper acetate, and the product is centrifugally washed and dried to obtain copper nitride powder with a cubic shape.
[0116] B. Weigh 3 mg of the copper nitride powder obtained in step A and evenly disperse it into 1 ml of absolute ethanol, add 6 μL of Nafion solution with a mass fraction of 5% to the mixed dispersion, ultrasonicate for 10 minutes to disperse evenly, and use 10 μL Use a pipette gun to spread the mixture evenly on 1*0.5cm -2 Gas diffusion conductive substrate (in this example, preferably gas diffusion carbon fiber paper is used as the conductive substrate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com