Preparation method and application of iso-phosphorus-manganese-iron-ore type iron phosphate
A technology based on iron phosphate and iron ore, applied in chemical instruments and methods, phosphorus compounds, recycling technology, etc., can solve the problems of high consumption of reagents, low consumption of reagents, and low product value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
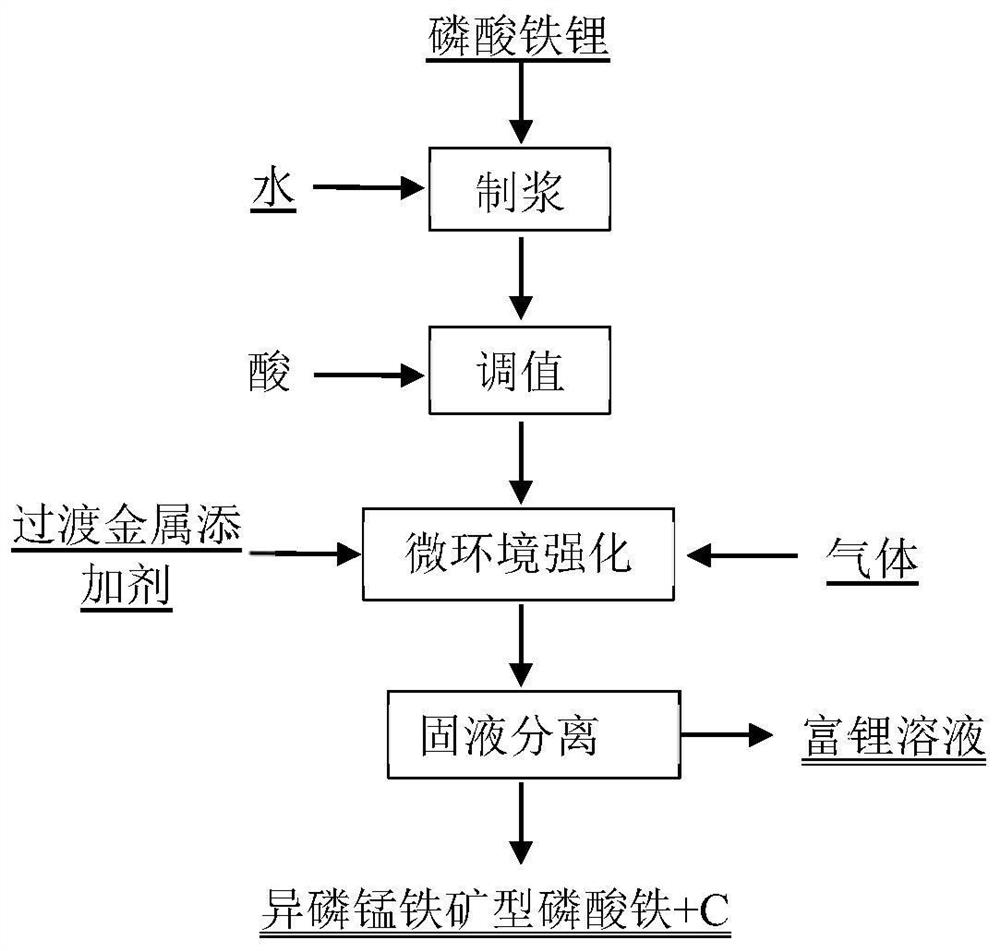
[0040] The preparation method of the present embodiment isophosphatite type iron phosphate comprises the following steps:
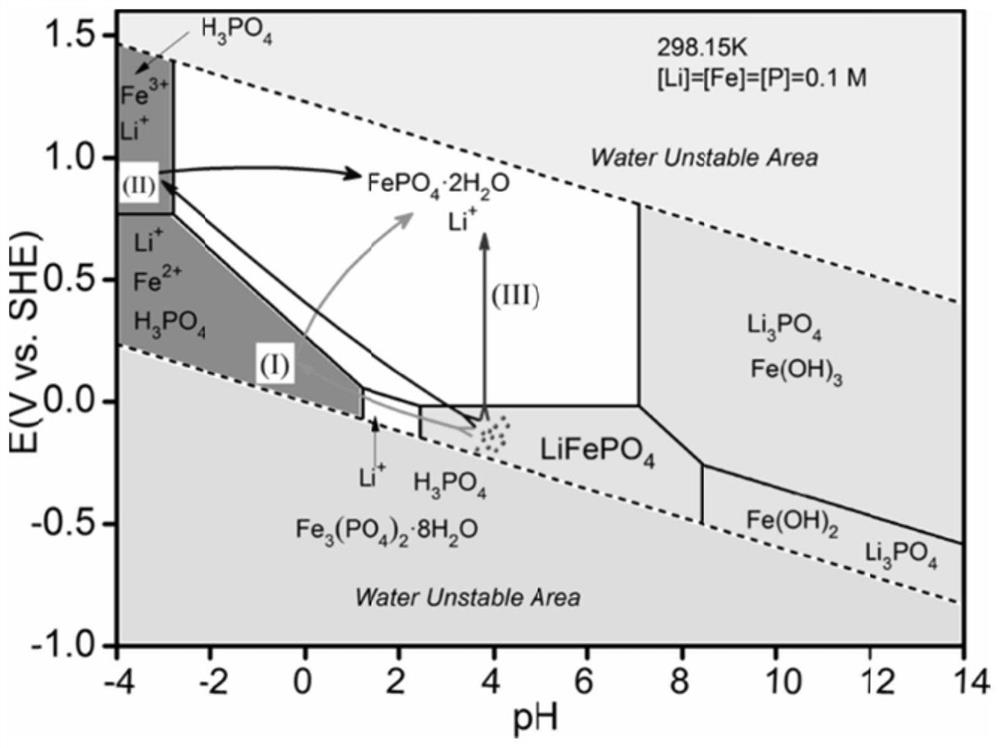
[0041] (1) Lithium iron phosphate and water are mixed according to the mass volume ratio of 50g / L, and then 2mol / L of hydrochloric acid is added to the slurry to maintain the pH of the leachate at 2 during the reaction to obtain an acidic lithium iron phosphate solution;
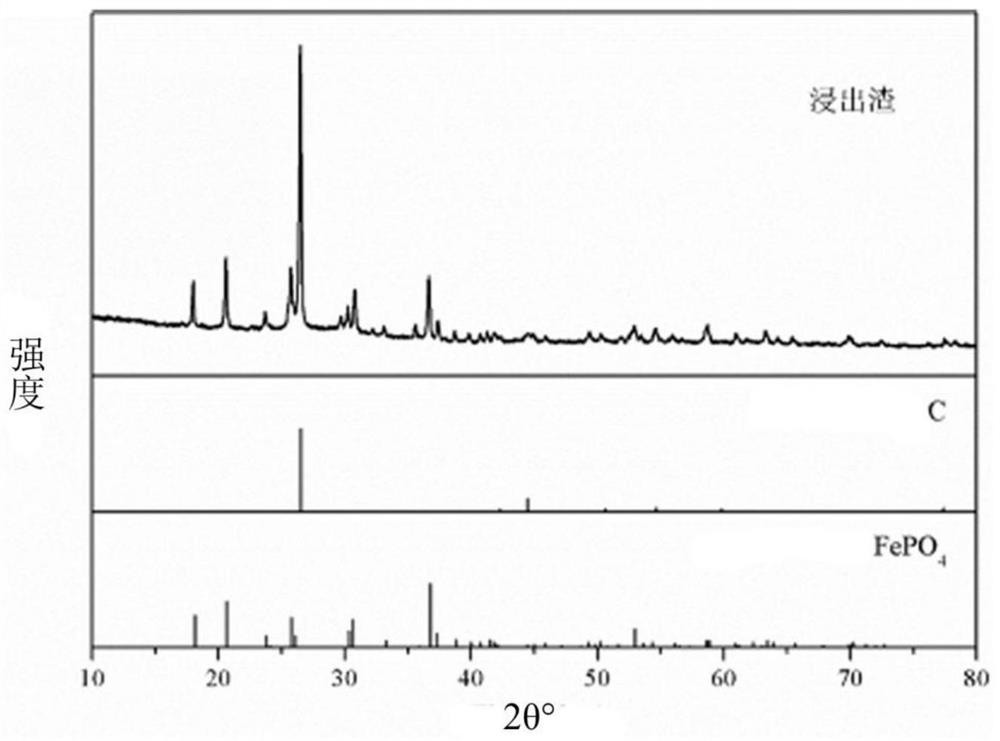
[0042] (2) Add tricobalt tetroxide into the acidic lithium iron phosphate solution, and introduce oxygen to form microbubbles with a diameter of 50um, leaching and reacting at 25°C for 240min, and filter to obtain the lithium-rich solution and Isostokite-type iron phosphate after the reaction is completed , the leaching rate of lithium in the leaching solution reaches 99.5%, and the contents of iron and phosphorus in the leaching solution are both lower than 0.1ppm.
Embodiment 2
[0044] The preparation method of the present embodiment isophosphatite type iron phosphate comprises the following steps:
[0045] (1) Lithium iron phosphate and water are mixed according to the mass volume ratio of 50g / L, and then 2mol / L of hydrochloric acid is added to the slurry to maintain the pH of the leachate at 6 during the reaction to obtain an acidic lithium iron phosphate solution;
[0046] (2) Add manganese dioxide into the acidic lithium iron phosphate solution, and introduce oxygen to form microbubbles with a diameter of 50um, leaching and reacting at 25°C for 240min, and filter to obtain the lithium-rich solution and Isostokite type after the reaction is completed. Iron phosphate, the leaching rate of lithium in the leaching solution reaches 93.9%, and the contents of iron and phosphorus in the leaching solution are both lower than 0.01ppm.
Embodiment 3
[0048] The preparation method of the present embodiment isophosphatite type iron phosphate comprises the following steps:
[0049] (1) Lithium iron phosphate and water are mixed according to the mass volume ratio of 400g / L, and then 2mol / L of hydrochloric acid is added to the slurry to maintain the pH of the leachate at 2 during the reaction to obtain an acidic lithium iron phosphate solution;
[0050] (2) Add lithium nickel cobalt manganese oxide into the acidic lithium iron phosphate solution, and introduce oxygen to form microbubbles with a diameter of 50um, leaching and reacting at 25°C for 240min, and filter to obtain lithium-rich solution and ferromanganese isophosphorus after the reaction is completed Mineral-type iron phosphate, the leaching rate of lithium in the leach solution reaches 96.7%, and the contents of iron and phosphorus in the leach solution are both lower than 0.1ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com