Preparation method of palbociclib intermediate
A technology of intermediates and treatment methods, which is applied in the field of preparation of palbociclib intermediates, can solve problems such as high cost ratio, large amount of sewage, complex components, etc., and achieve reduction of solid waste discharge, reduction of three waste discharge, Emission reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
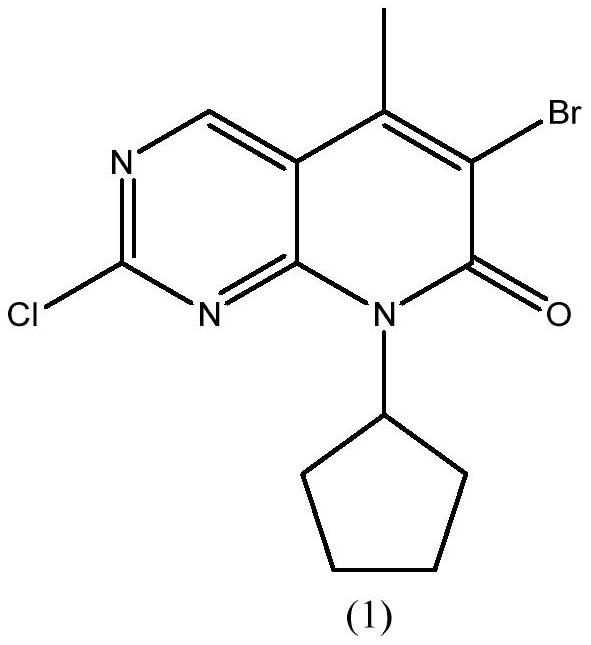
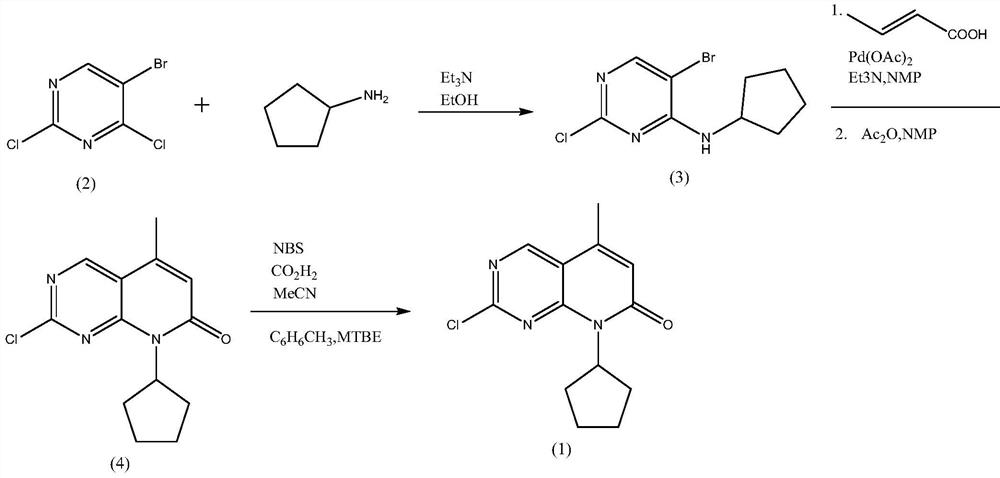
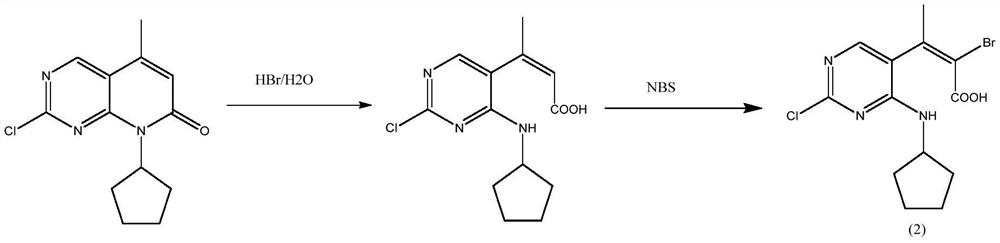
[0032] Example 1 Preparation of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one
[0033] 110 grams (0.42mol) of 2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one, 1100 milliliters of acetonitrile were added successively to the 2000 milliliter reaction flask, 2.7 grams of oxalic acid, 55 grams of acetic anhydride and 89.7 grams (0.50 mol) of NBS, heated to 60 ° C for 12 hours, the reaction was completed, cooled to 0 ~ 10 ° C, kept warm for 4 hours, filtered, washed with 100 ml of acetonitrile, and dried to obtain 140 grams of white solid , yield 98.1%, HPLC purity 98.5%.
[0034] The reaction mother liquor is distilled to near dryness, 1150ml of purple liquid, 50g of sodium hydroxide solid is added, stirred at room temperature for 2h, filtered to obtain a colorless filtrate, distilled at normal pressure to obtain 1105ml of acetonitrile, the GC detection of this acetonitrile is 99.9%, moisture (cal method) 0.2%.
[0035] Add 200ml of water...
example 2
[0036] Example 2: Preparation of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one
[0037] Add 110 grams (0.42mol) of 2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one successively to a 2000 ml reaction flask, and recover acetonitrile in 1100 ml , 2.7 g of oxalic acid, 55 g of acetic anhydride and 89.7 g (0.50 mol) of NBS, heated to 60 ° C for 12 hours, the reaction was completed, cooled to 0 ~ 10 ° C, kept warm for 4 h, filtered, washed with 100 ml of acetonitrile, dried to obtain a white solid 139 grams, yield 97.2%, HPLC purity 98.6%.
[0038] The reaction mother liquor was distilled to nearly dryness under atmospheric pressure, and 1130ml of purple liquid was added, 50g of sodium hydroxide solid was added, stirred at room temperature for 2h, and filtered to obtain a colorless filtrate, which was distilled under atmospheric pressure to obtain 1110ml of acetonitrile. 0.3%.
[0039]Add 200ml of water to dissolve the residue, adjust the p...
example 3
[0040] Example 3: Preparation of 6-bromo-2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one
[0041] Add 870 kg of acetonitrile, 110 kg of 2-chloro-8-cyclopentyl-5-methylpyrido[2,3-D]pyrimidin-7(8H)-one, 2.7 kg of oxalic acid, 55g of acetic anhydride and 89.7kg of NBS were heated up to 60°C and reacted for 12 hours. After the reaction was completed, the temperature was lowered to 0-10°C, kept warm for 4h, centrifuged, washed with 80kg of acetonitrile, and dried to obtain 138.4kg of white solid with a yield of 96.9%. HPLC 98.4% purity.
[0042] Transfer the reaction mother liquor to the recovery kettle, and distill at normal pressure to obtain a total of 920 kg of purple-red liquid. Add 45 kg of sodium hydroxide to recovery acetonitrile, stir at room temperature for 4 hours, press filter, filter the hydraulic pressure to the secondary distillation kettle, wash the filter residue with water, and discharge into the sewage station. After secondary atmospheric distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com