Nano-drug as well as preparation method and application thereof
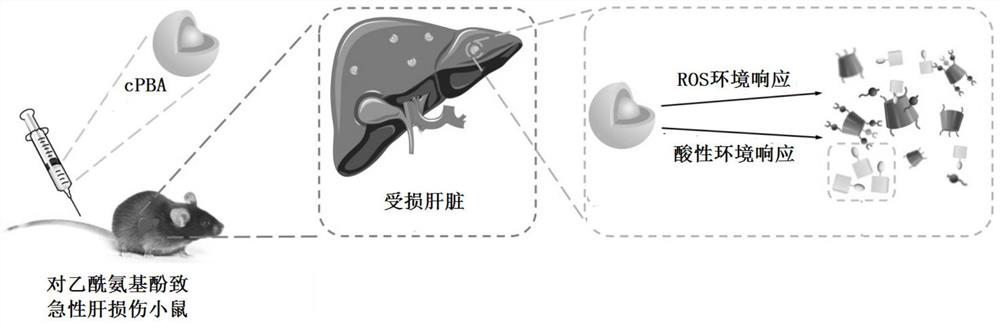
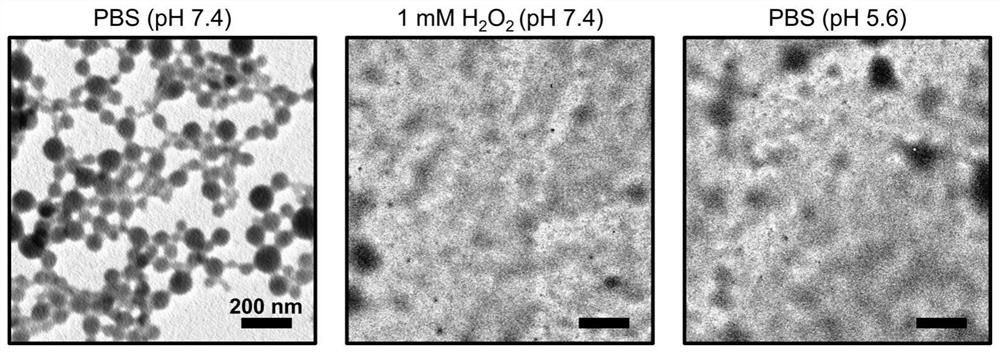
A technology of nano-drugs and derivatives, applied in the direction of nano-drugs, nanotechnology, nanotechnology, etc., can solve the problems of low degree of drug response, limited effect of drug molecule fixed-point controlled release, etc., to achieve improved bioavailability, good hydrogen peroxide Response ability and acidic environment response ability, effect of increasing accumulation concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The embodiment of the present application also provides a preparation method of the above-mentioned nano-medicine, the preparation method of the nano-medicine comprises the following steps:
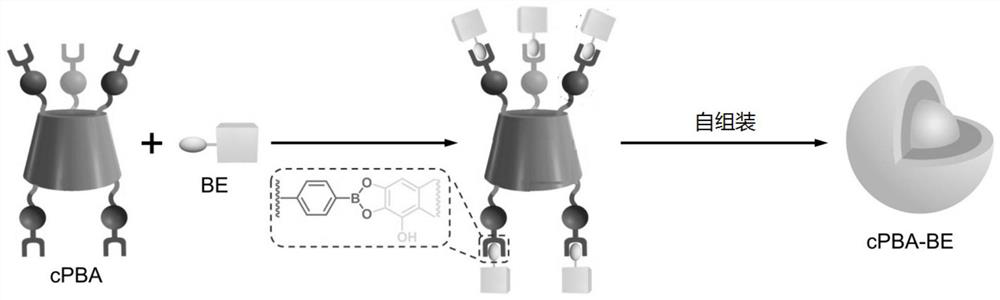
[0047] S01. Provide a cyclodextrin derivative and a polyhydroxy compound, the surface of the cyclodextrin derivative is modified with boronic acid groups, and the polyhydroxy compound contains at least two hydroxyl groups that are ortho or meta to each other;
[0048] S02, reacting the cyclodextrin derivative and the polyhydroxy compound, so that the hydroxyl group is connected to the boronic acid group to obtain a reaction product;
[0049] S03, dispersing the reaction product in an aqueous solution to assemble to form a nano drug.
[0050] Wherein, the types and properties of the cyclodextrin derivatives and polyols in step S01 refer to the cyclodextrin derivatives and polyols mentioned above, and will not be repeated here to save space.
[0051] Specifically, in step S02, the c...
Embodiment 1
[0064]In this example, a nanomedicine was prepared using 4-hydroxymethylphenylboronic acid-modified hydroxypropyl-β-cyclodextrin as a drug carrier and baicalein as a drug molecule. The preparation method includes the following steps:
[0065] 1. Preparation of 4-hydroxymethylphenylboronic acid-modified hydroxypropyl-β-cyclodextrin (cPBA)
[0066] Dissolve 199 mg of 4-hydroxymethylphenylboronic acid (PBA) and 213 mg of N,N'-carbonyldiimidazole (CDI) in anhydrous dichloromethane (DCM), stir for 1 hour and add 20 mL of dichloromethane and 30 mL of Deionized water was used for extraction, the organic phase was washed with saturated sodium chloride and dried with anhydrous sodium sulfate, and the organic phase was removed by rotary evaporation to obtain a CDI-activated PBA derivative (PBA-CDI). Then, under nitrogen protection, 725 mg of injection-grade hydroxypropyl-β-cyclodextrin (HP-β-CD) and 347 mg of PBA-CDI were dissolved in anhydrous dimethylformamide (DMF), and 250 μL of Af...
Embodiment 2
[0070] In this example, hydroxypropyl-β-cyclodextrin modified with 4-hydroxymethylphenylboronic acid is used as the drug carrier, and quercetin is used as the drug molecule to prepare a nano drug. The difference between the preparation method and Example 1 It mainly lies in the preparation steps of nanomedicine, including:
[0071] Dissolve 163mg of quercetin and 200mg of cPBA in 4mL of anhydrous DMSO containing molecular sieves to react overnight, slowly add the reaction solution into PBS buffer, then place the mixture in a dialysis bag, dialyze in deionized water, and then carry out Freeze dried.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com