Anti-human Trop-2 antibody and application thereof
An antibody and half-antibody technology, applied in the direction of antibodies, applications, antibody mimetics/scaffolds, etc., can solve the problem of less varieties of anti-Trop-2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Preparation of anti-human Trop-2 antibody hybridoma cells
[0098] Immunization: adopt human Trop-2 recombinant protein (sequence number: NP_002344.2, 1aa-274aa) to immunize Balb / c mice, and use human Trop-2-his recombinant protein (sequence number: NP_002344.2, 1aa-274aa) to coat ) 96-well ELISA plate to detect the serum titer by ELISA; the mice whose serum titer reached the fusion requirement were used for the next step of cell fusion.
[0099] Cell fusion and hybridoma preparation: select mice whose titer reaches the requirement, and carry out shock immunization. After 3 days, the spleen of the mouse is aseptically taken, and B lymphocyte suspension is prepared, which is mixed with SP2 / 0 myeloma cells at a ratio of 4:1. , the two cells were fused under the action of PEG. After the fused cells were resuspended in HAT medium, they were divided into 96-well cell culture plates. Set at 37°C, 5% CO 2 Cultured in an incubator.
Embodiment 2
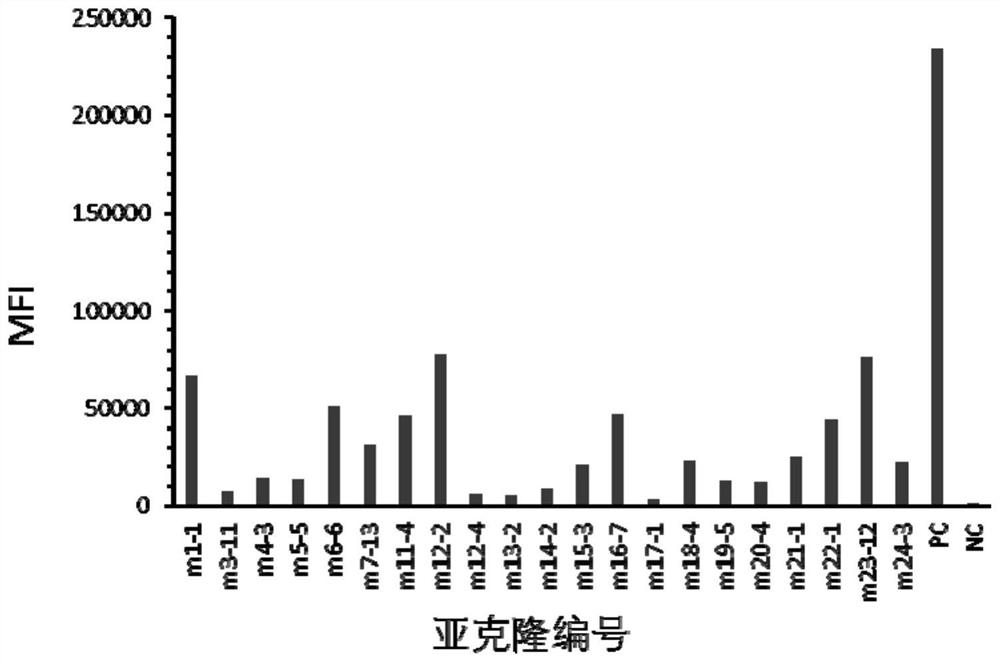
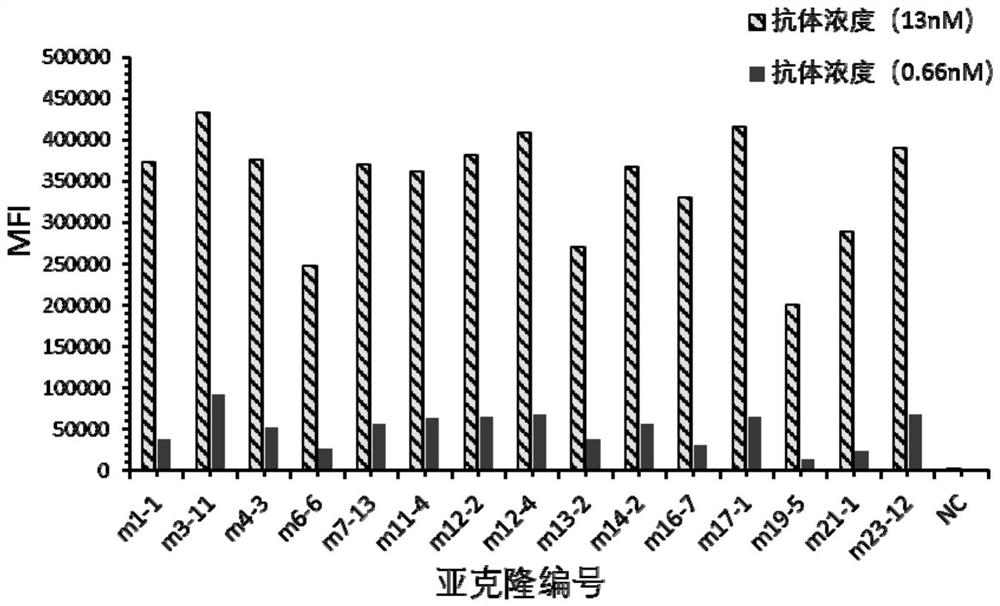
[0100] Example 2 Screening of anti-human Trop-2 antibody-positive hybridoma cell lines
[0101] 1. Positive Hybridoma Binding Screening
[0102] 10-14 days after fusion, coat the microtiter plate with human Trop-2-his recombinant protein (serial number: NP_002344.2, 1aa-274aa) (20ng / ml), overnight at 4°C; wash three times with PBS, wash with Block with 4% skim milk powder-PBS, room temperature, 1 hr; wash three times with PBS, add hybridoma clone culture supernatant, room temperature, 1 hr. The following controls were set up: (1) positive control (PC): mouse serum after immunization (diluted with PBS 1:1000); (2) negative control (NC): fusion wells without cell growth. Wash three times with PBST (0.05% Tween-PBS), wash twice with PBS, add HRP-goat anti-mouse IgG (Fcγ), 37°C, 0.5hr; then wash three times with PBST (0.05% Tween20-PBS), Add TMB chromogenic solution, protect from light for 15-30 minutes, add ELISA stop solution to terminate the reaction; read the A450 value wi...
Embodiment 3
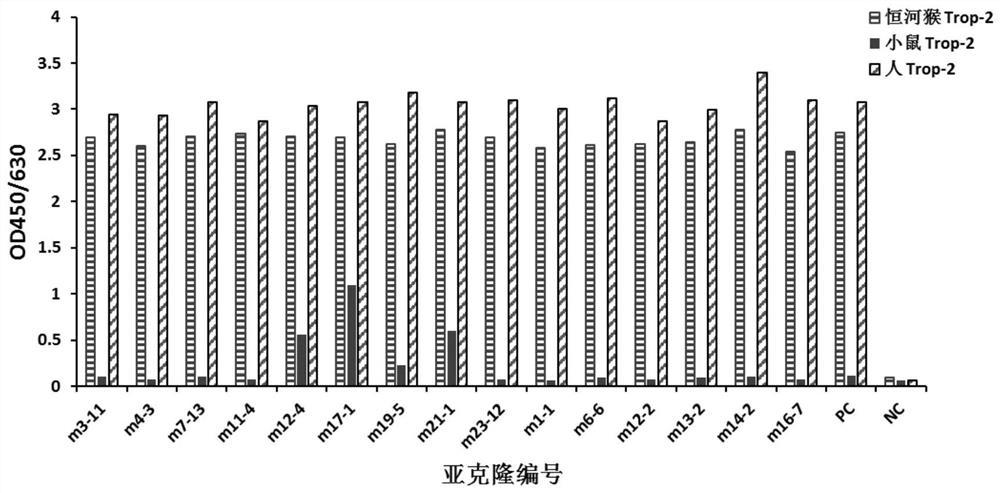
[0111] Example 3 Sequence determination of murine anti-human Trop-2 antibody
[0112] After the hybridoma cells m3-11, m4-3, m11-4, m17-1, m23-12 secreting anti-human Trop-2 antibody were expanded and cultured, they were cultured with Mouse Monoclonal Antibody IgG Subclass Test Card (Cat.: A12403, VicNovo ) and Mouse Monoclonal Antibody Light / Heavy Chain Test Card (Cat.: A12401, VicNovo) for subtype detection in accordance with the reagent operating procedures, subtype identification: heavy chain is IgG1, light chain is Kappa chain, m3-11, m4 -3, m11-4, m17-1, m23-12 antibody gene cloning provides basis.
[0113] The m3-11, m4-3, m11-4, m17-1, m23-12 hybridoma cells were extracted from the total RNA according to the instructions of the TRIzol kit (Cat.: 15596026, Invitrogen); using M-MuLV reverse transcriptase (Cat.: M0253S, NEB) to reverse transcribe the total RNA of hybridoma cells into cDNA; use degenerate primers (refer to the book [Dong Zhiwei, Wang Yan. Antibody Engin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com