Preparation method of soluble florfenicol powder
A technology for soluble florfenicol and florfenicol, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve poor palatability, reduce animal appetite, and cannot cover up The problem of drug bitterness, etc., achieves the effect of high inclusion rate, improved bioavailability, and good inclusion effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of soluble florfenicol powder, this soluble florfenicol powder is made from the raw material of following mass parts:
[0041] Florfenicol 5g, β-cyclodextrin 2g, polyethylene glycol 3g, silicon dioxide 2g, acid excipient 2g, bactericidal ingredient 2g.
[0042] Wherein, the acidic auxiliary material includes the following raw material components: 1 g of anhydrous citric acid and 1 g of anhydrous glucose.
[0043] Wherein, the composite antioxidant includes the following raw material components: 0.5 g of trimethoprim lactate and 1.5 g of sulfanilamide powder.
[0044] The preparation for soluble florfenicol powder comprises the following steps:
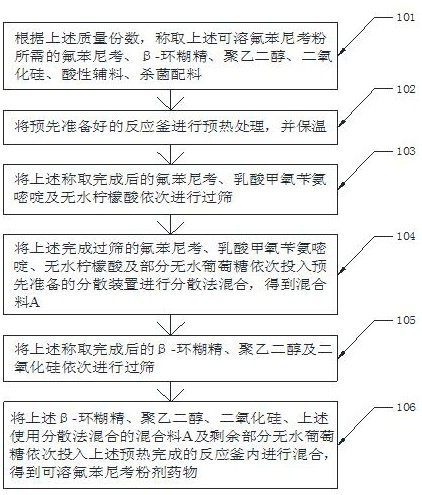
[0045] S101, according to the above mass parts, weigh 5 g of florfenicol, 2 g of β-cyclodextrin, 3 g of polyethylene glycol, 2 g of silicon dioxide, 2 g of acidic auxiliary materials, sterilizing Ingredients 2g;
[0046] S102, preheating the pre-prepared reactor and keeping it warm;
[0047] S103, sieve 5 g of florfen...
Embodiment 2
[0052] A kind of soluble florfenicol powder, this soluble florfenicol powder is made from the raw material of following mass parts:
[0053] Florfenicol 10g, β-cyclodextrin 3.5g, polyethylene glycol 5g, silicon dioxide 4g, acidic excipient 3.5g, bactericidal ingredient 2.5g.
[0054] Wherein, the acidic auxiliary material includes the following raw material components: 1.5 g of anhydrous citric acid and 2 g of anhydrous glucose.
[0055] Wherein, the composite antioxidant includes the following raw material components: 0.75 g of trimethoprim lactate and 1.75 g of sulfanilamide powder.
[0056] The preparation of this soluble florfenicol powder comprises the following steps:
[0057] S101, according to the above mass parts, weigh 10 g of florfenicol, 3.5 g of β-cyclodextrin, 5 g of polyethylene glycol, 4 g of silicon dioxide, and 3.5 g of acidic auxiliary materials required for the above-mentioned soluble florfenicol powder , 2.5g of sterilizing ingredients;
[0058] S102, p...
Embodiment 3
[0064] A kind of soluble florfenicol powder, this soluble florfenicol powder is made from the raw material of following mass parts:
[0065] Florfenicol 15g, β-cyclodextrin 5g, polyethylene glycol 7g, silicon dioxide 6g, acid excipient 5g, bactericidal ingredient 3g.
[0066] Wherein, the acidic auxiliary material includes the following raw material components: 2 g of anhydrous citric acid and 3 g of anhydrous glucose.
[0067] Wherein, the composite antioxidant includes the following raw material components: 1 g of trimethoprim lactate and 2 g of sulfanilamide powder.
[0068] The preparation of this soluble florfenicol powder comprises the following steps:
[0069] S101, according to the above mass parts, weigh 15 g of florfenicol, 5 g of β-cyclodextrin, 7 g of polyethylene glycol, 6 g of silicon dioxide, 5 g of acidic auxiliary materials, and sterilizing Ingredients 3g;
[0070] S102, preheating the pre-prepared reactor and keeping it warm;
[0071] S103, sieve 15 g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com