A kind of bremelanotide oral pharmaceutical composition and application thereof
A composition and drug technology, applied in the field of bremelanotide oral pharmaceutical composition and its application, and polypeptide drug oral pharmaceutical composition, can solve the complex preparation process, unexplained influence, unexplained gastric absorption, etc. problems, to achieve the effect of improving the degree of absorption, avoiding potential dangers, and solving poor compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
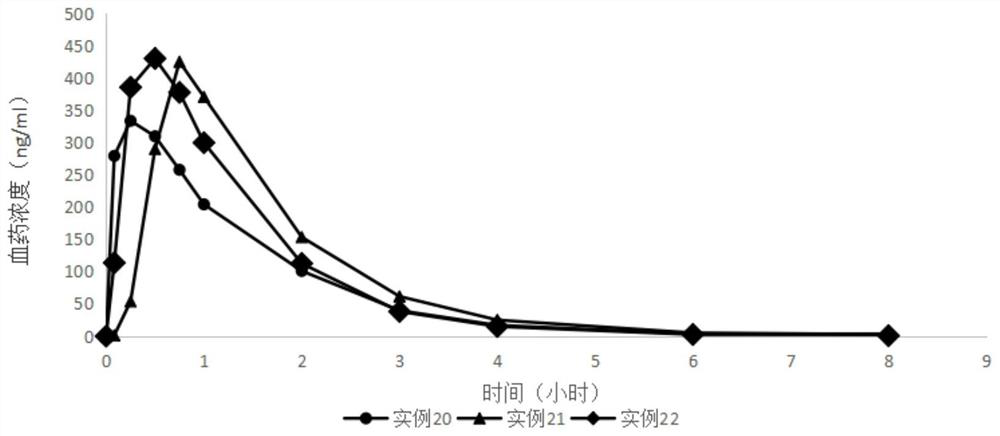
Examples
Embodiment 1
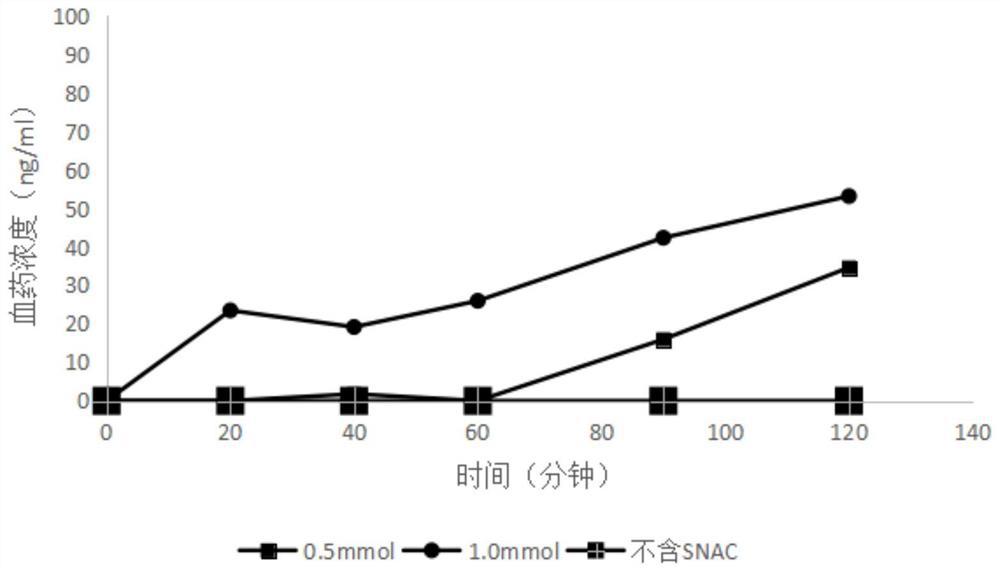
[0102] Rats were fasted for 16 hours before administration, but had free access to water. After intraperitoneal injection of anesthesia, the rats were placed under a heating lamp to keep the body temperature at about 37°C, and the intestine was exposed through a midline abdominal incision. After the bile duct was ligated, the intestinal tract was flushed with phosphate buffer (pH 7.4), and the remaining buffer was vented with air. Both ends of the small intestine were cannulated with polyethylene tubing. Perfuse the drug solution containing the absorption enhancer or the solution containing only the drug, the volume is 200ml, the solution is Krebs-Ringer solution, and the preparation method is to weigh 7.8g of NaCl, 0.35g of KCl, and 0.35g of CaCl 2 0.37g, NaHCO3 1.37g, NaH 2 PO 4 0.32g, MgCl 2 0.02g, glucose 1.4g, dissolved in a small amount of water, in which CaCl 2 Add dropwise after dissolving separately, add glucose immediately after use, dilute to 1L with purifie...
Embodiment 2
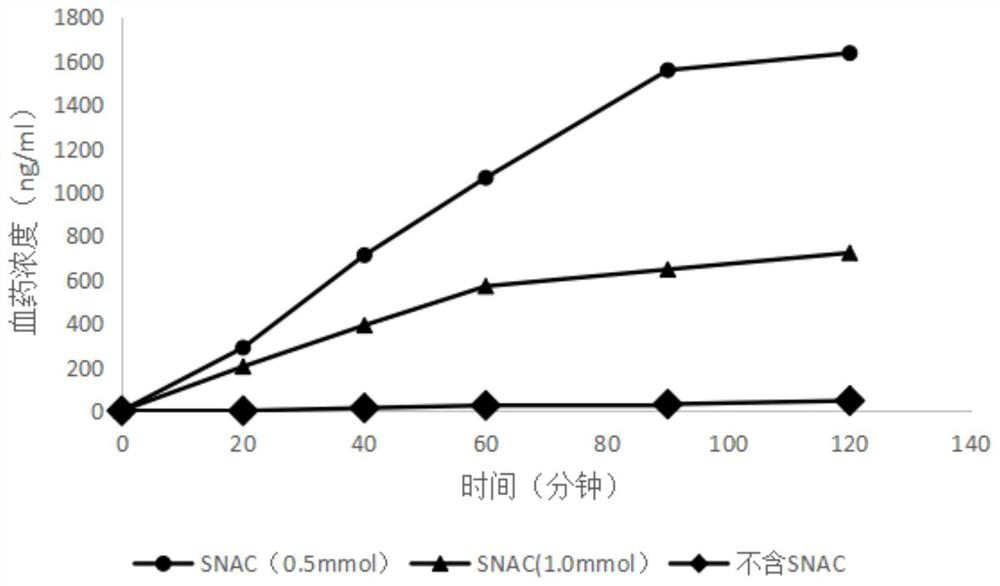
[0108] Rats were fasted for 16 hours before administration, but had free access to water. After intraperitoneal injection of anesthesia, the rats were placed under a heating lamp to keep the body temperature at about 37°C, and the stomach was exposed through a midline abdominal incision. Incision was made at both ends of the stomach, polyethylene tubes were inserted at both ends of the stomach, the stomach was flushed with phosphate buffer (pH 7.4), and the remaining buffer was vented with air. Perfuse the drug solution containing the absorption enhancer or the solution containing only the drug, the volume is 200ml, the solution is Krebs-Ringer solution, and the preparation method is the same as in Example 1. The perfusion solution was maintained at 37°C and the solution administered through the catheter was warmed and circulated. The jugular vein was exposed, and 0.3 ml blood samples were collected by direct puncture of a heparin syringe at 20, 40, 60, 90, and 120 minutes af...
Embodiment 3
[0116] Prepare artificial gastric juice (refer to the preparation method of the United States Pharmacopoeia) and artificial intestinal juice (refer to the preparation method of phosphate buffer solution with a pH value of 6.8 containing trypsin included in the Chinese Pharmacopoeia 2020 edition). Weigh an appropriate amount of bremelanotide, dissolve it with artificial gastric juice and artificial intestinal juice respectively to form a 0.1 mg / ml solution, and place it in a 37°C water bath. The content of bremelanotide in artificial gastric juice and artificial intestinal juice was detected by high performance liquid chromatography. Detection method: high performance liquid chromatography, C 18 Column, detection wavelength 215nm; column temperature 30°C; flow rate 1.0ml / min; injection volume: 20ul; sample concentration 0.1mg / ml; mobile phase A contains 1% trifluoroacetic acid in acetonitrile solution, mobile phase B contains 1% Aqueous solution of fluoroacetic acid; see Table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com