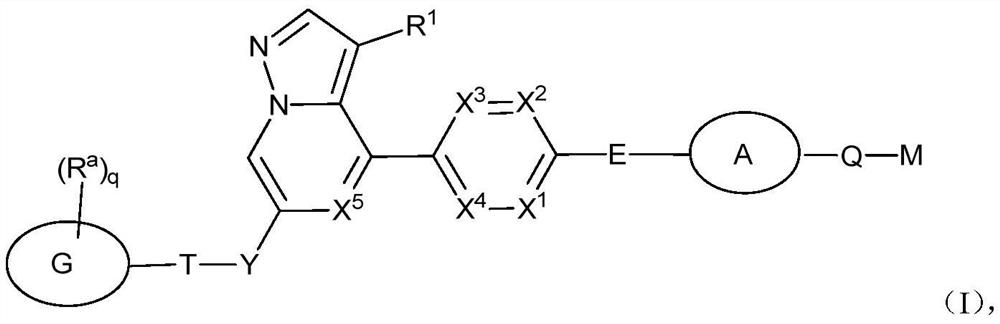
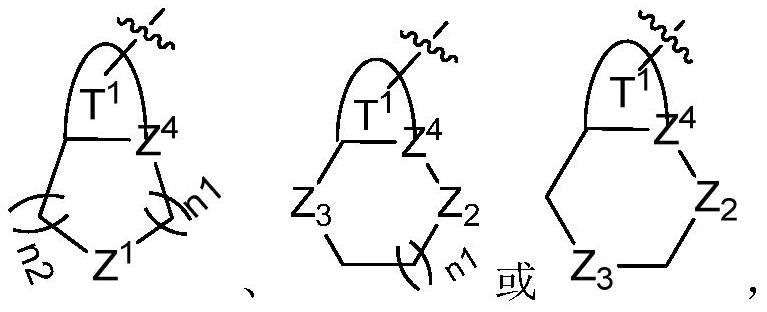
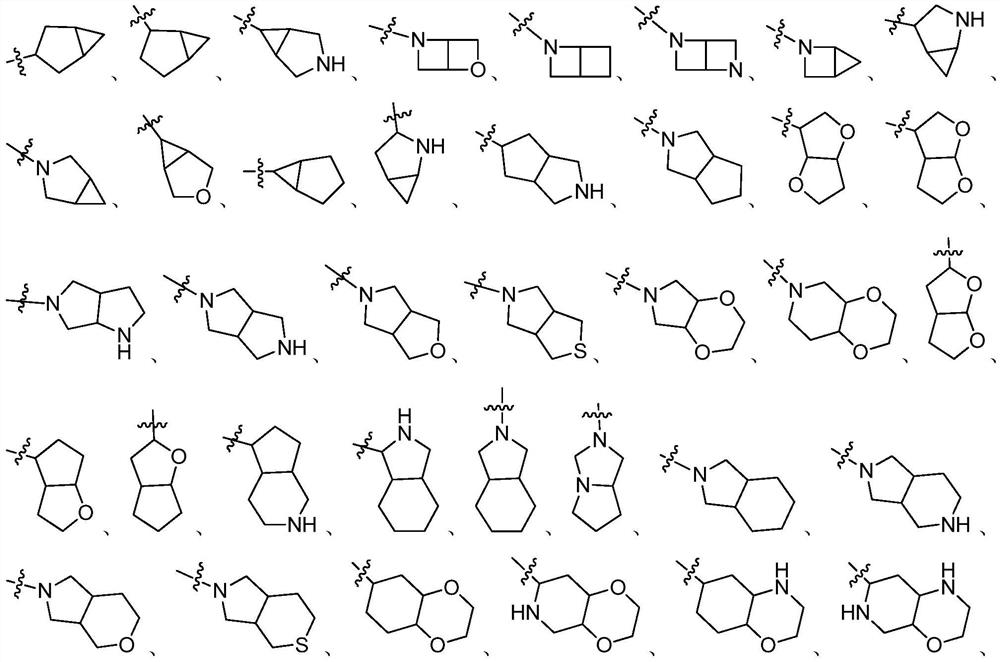
RET inhibitor, pharmaceutical composition and application thereof
A technology of compounds and solvates, applied in the field of RET inhibitors, their pharmaceutical compositions and their uses, can solve problems such as administration and toxicity, and achieve the effects of promoting side effects, good inhibitory effect, and good inhibitory selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0491]Example 1: 4-(6-(6-((6-methoxypyridin-3-yl)methyl)-3,6-diazabicyclo[3.1.1]heptane-3-yl)pyridine -3-yl)-6-(2-(tetrahydro-2H-[1,4]dioxin[2,3-c]pyrrol-6(3H)-yl)ethoxy)pyrazolo[1, Synthesis of 5-a]pyridine-3-carbonitrile
[0492]
[0493] Step 1: Hexahydro-2H-[1,4]dioxine[2,3-c]pyrrole hydrochloride
[0494] Add tetrahydro-2H-[1,4]dioxin[2,3-c]pyrrole-6(3H)-tert-butyl carboxylate (2230mg, 9.73mmol), HCl / EA(4N) in sequence to 100mL one-mouth bottle (18 mL), stirred at rt for 8 hours. TLC monitors the complete reaction of raw materials, spins dry and directly puts into the next reaction. LC-MS: (ESI-MS): m / z=130.1[M+H] + .
[0495] Step 2: 6-(2-Chloroethyl)hexahydro-2H-[1,4]dioxino[2,3-c]pyrrole
[0496] Add hexahydro-2H-[1,4]dioxine[2,3-c]pyrrole hydrochloride (4.5mmol, 740mg, acetone (15mL), K 2 CO 3 (2030 mg, 14.7 mmol), stirred overnight at rt. The reaction liquid was filtered with celite, washed with EA (15mL×3), concentrated, and subjected to silica gel colum...
Embodiment 2
[0499] Example 2: 6-(2-(5-hydroxyl-5-methylhexahydrocyclopenta[c]pyrrol-2(1H)-yl)ethoxy)-4-(6-(6-((6 -Methoxypyridin-3-yl)methyl)-3,6-diazabicyclo[3.1.1]heptane-3-yl)pyridin-3-yl)pyrazole[1,5-a] Synthesis of Pyridine-3-carbonitrile
[0500]
[0501] Step 1: tert-butyl N-formate-5-hydroxy-5-methylhexahydrocyclopentane[c]pyrrole
[0502] 0°C, N-tert-butyl formate-hexahydro-5-oxocyclopenta[c]pyrrole (1.00g, 4.44mmol) was dissolved in anhydrous THF (10mL), slowly added THF solution of methylmagnesium bromide (3mL, 9mmol, 3mol / L), naturally rose to room temperature and stirred for 1h. The reaction was monitored by TLC (PE / EA (v / v)=1 / 1, Rf=0.32), and the reaction of the starting material was complete. saturated NH 4 Quench the reaction with Cl solution, stop the reaction, filter with suction, concentrate, perform silica gel column chromatography, eluent PE / EA (v / v)=10 / 1-3 / 1, and obtain 0.71 g of a yellow-brown solid, which is the target product , yield 66%. 1 H NMR (400MHz,...
Embodiment 3
[0509] Example 3: 6-(2-(5-methoxyhexahydrocyclopenta[c]pyrrol-2(1H)-yl)ethoxy)-4-(6-(6-((6-methoxy Basepyridin-3-yl)methyl)-3,6-diazabicyclo[3.1.1]heptane-3-yl)pyridin-3-yl)pyrazol[1,5-a]pyridine-3 -Synthesis of forminonitrile
[0510]
[0511] Step 1: tert-butyl N-formate-5-hydroxyhexahydrocyclopentane[c]pyrrole
[0512] In an ice-water bath, LiAlH 4 (0.35g, 9.2mmol) was slowly added to anhydrous THF (15mL), after stirring at low temperature for 15min, slowly added N-tert-butyl formate-hexahydro-5-oxocyclopenta[c]pyrrole (1.03g, 4.57 mmol), naturally rose to room temperature and stirred for 2h. The reaction was monitored by TLC (PE / EA (v / v)=4 / 1, iodine fumigation, Rf=0.18), and the reaction of the raw material was complete. saturated Na 2 SO 4 The solution was quenched, filtered with suction, and concentrated to obtain 0.89 g of brown-yellow viscous liquid (yield 86%). 1 H NMR (400MHz, CDCl 3 )δ4.34-4.23(m,1H),3.54-3.45(m,2H),3.38-3.28(m,2H),2.81-2.64(m,1H),2.62-2.52...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com