Neovascular-targeting contrast medium composition and method for preparing same
An angiogenesis and contrast agent technology, which can be used in preparations for in vivo experiments, biomaterial analysis, chemical instruments and methods, etc. strength, excellent tissue permeability, and biostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1. Preparation of contrast agents for targeting angiogenesis
[0057] 2,3,5,6-tetrafluorophenyl-6-[ 18 Fluoro]-flunicotinate (2,3,5,6-tetrafluorophenyl-6-[ 18 F]-fluoronicotinate).
[0058] The 2,3,5,6-tetrafluorophenyl-6-[ 18 Fluoro]-flunicotinate and 0.2ml of 0.1M sodium bicarbonate (NaHCO 3 ) aqueous solution was reacted together at normal temperature for 10 minutes, and after removing unreacted substances using a size exclusion column (size exclusion column) (PD-10 type, GE Healthcare Company), finally prepared 43 mCi 18 Fluoro-Nanobodies.
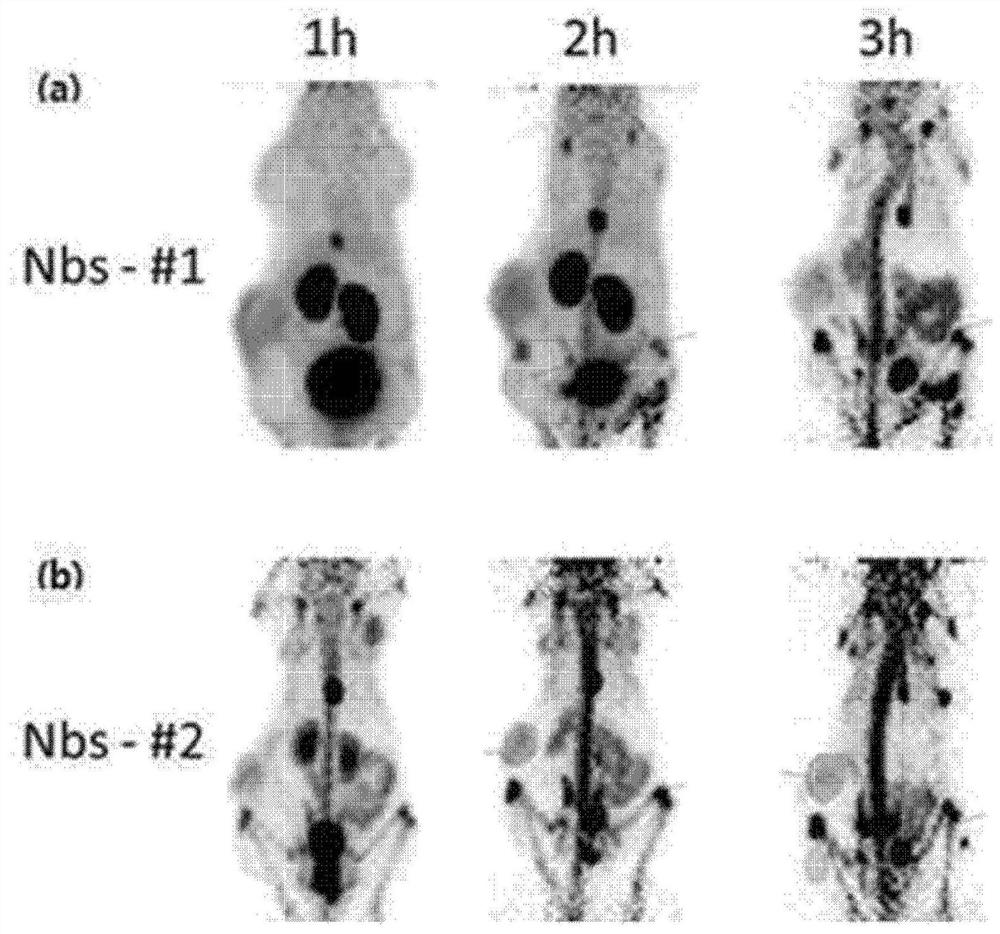
[0059] Prepared using the Nanobody given by him that contains lysine (Lysine) and hexahistidine tag (His6) in the base sequence 1 18 Fluorine-nanobodies are abbreviated as Nbs-#1 and Nbs-#2, respectively.
[0060] Table 1 shows the results of confirming the radioactivity (radioactivity) of 300 µL aliquots sequentially collected using a PD-10 type column.
[0061] Table 1
[0062] vial Activity (uCi / 30...
experiment example 1
[0063] Experimental example 1. Confirmation of labeling rate of contrast agent for targeting angiogenesis
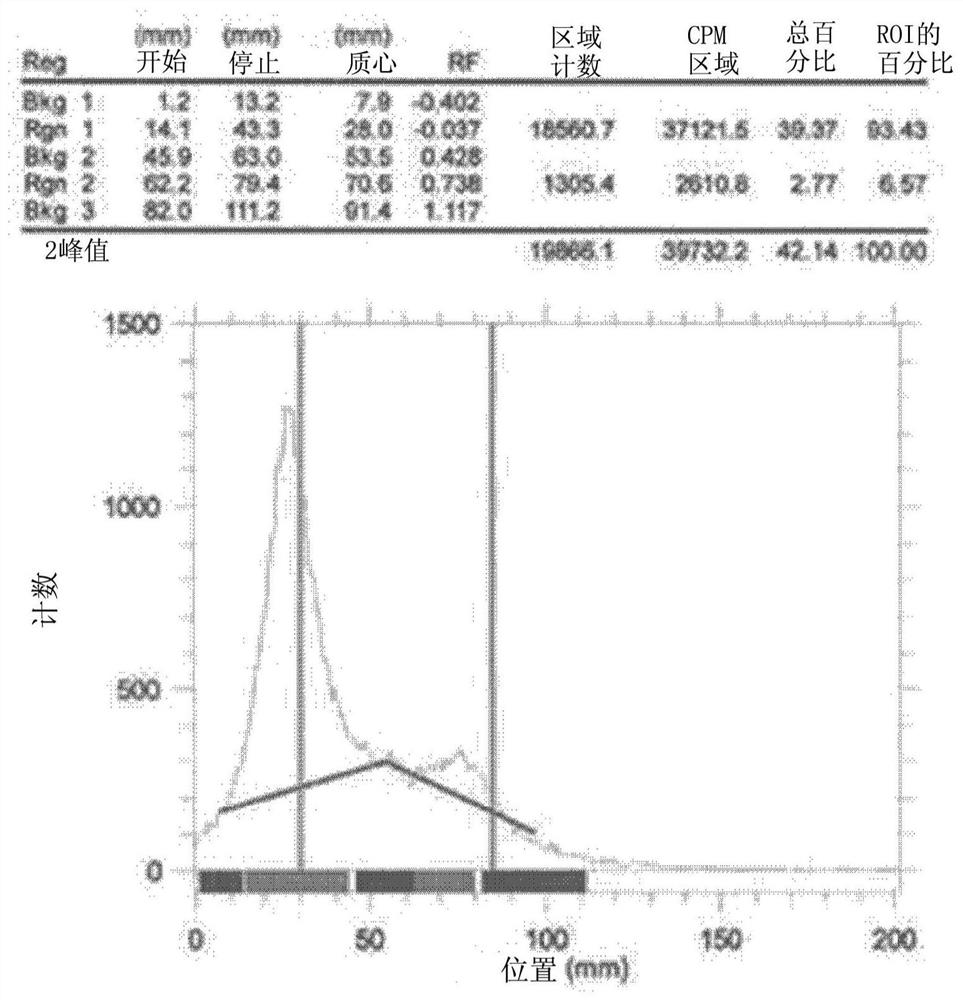
[0064] Utilize radioactive thin-layer chromatography (Radio-TLC) to confirm that prepared in embodiment 1 18 The radiochemical yield of fluoro-nanobodies, the results of which are as follows figure 1 shown.
[0065] Such as figure 1 As shown, the 2,3,5,6-tetrafluorophenyl-6-[ 18When the fluoro]-flunicotinate and the nanobody react for 10 minutes at normal temperature, the radiochemical yield is over 93%.
experiment example 2
[0066] Experimental example 2. Confirmation of the angiogenesis targeting of the present invention at the tumor site 18 Fluoro-Nanobody Alpha v beta 3 Integrin targeting effects
[0067] In order to confirm the contrast effect of the contrast agent composition of the present invention prepared in the above-mentioned Example 1 in the tumor mouse animal model, the following experiment was carried out.
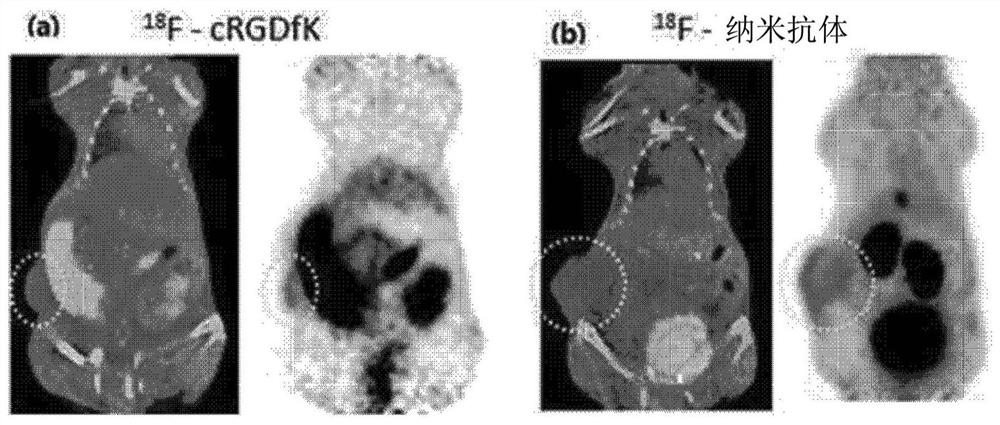
[0068] Will 18 F-cRGDfk was used as a positive control to confirm that α v beta 3 Integrin targeting effects.
[0069] Specifically, U87-MG cells (U87-MG cells) were xenografted (inoculation) into athymic nude mice (female, 6-8 weeks old) to prepare animal models. When the tumor grows to 0.8cm-1cm in size, inject (1mg / kg) from the tail vein and analyze the contrast effect according to the time. The result is as figure 2 and image 3 shown.
[0070] Such as figure 2 indicated, intravenous infusion 18 F-cRGDfk or 18 After the fluorine-nanobody, one hour later, it wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com